Contribution of Coronavirus-Specific Immunoglobulin G Responses to Complement Overactivation in Patients with Severe Coronavirus Disease 2019
- PMID: 35267024
- PMCID: PMC8992249
- DOI: 10.1093/infdis/jiac091
Contribution of Coronavirus-Specific Immunoglobulin G Responses to Complement Overactivation in Patients with Severe Coronavirus Disease 2019
Abstract
Background: Excessive complement activation has been implicated in the pathogenesis of coronavirus disease 2019 (COVID-19), but the mechanisms leading to this response remain unclear.
Methods: We measured plasma levels of key complement markers, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA and antibodies against SARS-CoV-2 and seasonal human common cold coronaviruses (CCCs) in hospitalized patients with COVID-19 of moderate (n = 18) and critical severity (n = 37) and in healthy controls (n = 10).
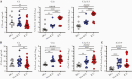
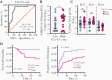
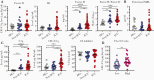
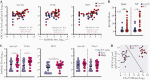
Results: We confirmed that complement activation is systemically increased in patients with COVID-19 and is associated with a worse disease outcome. We showed that plasma levels of C1q and circulating immune complexes were markedly increased in patients with severe COVID-19 and correlated with higher immunoglobulin (Ig) G titers, greater complement activation, and higher disease severity score. Additional analyses showed that the classical pathway was the main arm responsible for augmented complement activation in severe patients. In addition, we demonstrated that a rapid IgG response to SARS-CoV-2 and an anamnestic IgG response to the nucleoprotein of the CCCs were strongly correlated with circulating immune complex levels, complement activation, and disease severity.
Conclusions: These findings indicate that early, nonneutralizing IgG responses may play a key role in complement overactivation in severe COVID-19. Our work underscores the urgent need to develop therapeutic strategies to modify complement overactivation in patients with COVID-19.
Keywords: COVID-19; SARS-CoV-2; and antibodies; classical pathway; common cold coronaviruses; complement system.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. U. B. N. reports grants from the National Science Foundation, and Texas A&M Institute of Data Science, and book royalties from Springer, unrelated to the current work. J. M. reports grants from the National Institutes of Health (NIH), USAID, Gilead Sciences, and Janssen Pharmaceuticals; serves or has served as a consultant for Gilead Sciences, Accelevir Diagnostics, and Xi’an Yufan Biotechnologies; owns share options in Co-Crystal Pharmaceuticals and Infectious Diseases Connect; and is a shareholder of Abound Bio. His holdings in Co-Crystal Pharmaceuticals, Infectious Diseases Connect, and Abound Bio are unrelated to the current work. A. M. reports grants from the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases, NIH during the conduct of the study; B. J. M. reports grants from Bayer Pharmaceuticals, the Translational Breast Cancer Research Consortium, and the UPMC Learning While Doing Program, during the conduct of the study; consulting fees from Boehringer Ingelheim, the VeraMedica Institute; and the Patient-Centered Outcomes Research Institute for the ACCOMPLISH Trial, outside the submitted work. F. S. reports grants from the National Institute of General Medical Sciences, NIH, during the conduct of the study. B. J. C. M. reports grants from NIH and Gilead Sciences during the conduct of the study. D. B. reports leadership roles as president and board chair of Epistemix, director of the Magee Women’s Research Institute, and member of the Allegheny County Board of Health, unrelated to the current work, and is also a shareholder of Epistemix, unrelated to the current work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

