Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity
- PMID: 35267461
- PMCID: PMC8909312
- DOI: 10.3390/cancers14051153
Diagnostic Performance of a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program According to Ambient Temperature and Humidity
Abstract
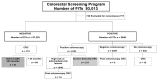
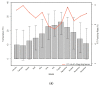
Exposure of the fecal immunochemical test (FIT) to different ambient temperatures and humidity is unavoidable in population-based screening programs in Southern European countries, and it could lead to a decrease in target colorectal lesions. The objective was to evaluate the effect of ambient temperature and humidity on the FIT sensitivity in a population-based screening program for colorectal cancer (CRC) using an ecological design. The retrospective cohort included individuals aged 50−69 years who participated in CRC screening (Barcelona) from 2010−2015, and were followed until 2017 to identify interval CRCs. The positivity rate, and detection rates for advanced polyps and CRC were compared according to ambient temperature, humidity, and quarters of the year. A positive FIT was defined as the detection of ≥20 μg Hb/g in feces. The monthly ambient temperature and humidity were recorded on the day that the FIT was performed. In total, 92,273 FIT results from 53,860 participants were analyzed. The FIT positivity rate was lower at >24 °C than at ≤24 °C (p = 0.005) but was not affected by humidity. The temperature’s impact on positivity did not lead to a decrease in the FIT detection rate for advanced neoplasia or the interval cancer detection rate in a program where the samples were refrigerated until the analysis and screening invitations were discontinued in July and August.
Keywords: fecal immunochemical test; humidity; interval colorectal cancer; screening program; temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ponti A., Anttila A., Ronco G., Senore C. Report on the Implementation of the Council Recommendation on Cancer Screening. International Agency for Research on Cancer; Lyon, France: 2017.
-
- Alhomoud S., Blom J., Bretthauer M., Bulliard J.L., Corley D., Garcia Martinez M., Hoffmeister M., Hultcrantz R., Lansdorp-Vogelaar I., Nagtegaal I., et al. Colorectal Cancer Screening. Volume 17 IARC Working Group on the Evaluation of Cancer-Preventive Interventions; Lyon, France: 2019.

