Evaluating Alternative Ramucirumab Doses as a Single Agent or with Paclitaxel in Second-Line Treatment of Locally Advanced or Metastatic Gastric/Gastroesophageal Junction Adenocarcinoma: Results from Two Randomized, Open-Label, Phase II Studies
- PMID: 35267477
- PMCID: PMC8909008
- DOI: 10.3390/cancers14051168
Evaluating Alternative Ramucirumab Doses as a Single Agent or with Paclitaxel in Second-Line Treatment of Locally Advanced or Metastatic Gastric/Gastroesophageal Junction Adenocarcinoma: Results from Two Randomized, Open-Label, Phase II Studies
Abstract
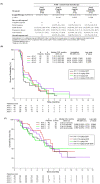
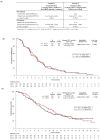
Studies JVDB and JVCZ examined alternative ramucirumab dosing regimens as monotherapy or combined with paclitaxel, respectively, in patients with advanced/metastatic gastric/gastroesophageal junction (GEJ) adenocarcinoma. For JVDB, randomized patients (N = 164) received ramucirumab monotherapy at four doses: 8 mg/kg every 2 weeks (Q2W) (registered dose), 12 mg/kg Q2W, 6 mg/kg weekly (QW), or 8 mg/kg on days 1 and 8 (D1D8) every 3 weeks (Q3W). The primary objectives were the safety and pharmacokinetics of ramucirumab monotherapy. For JVCZ, randomized patients (N = 245) received paclitaxel (80 mg/m2-D1D8D15) plus ramucirumab (8 mg/kg- or 12 mg/kg-Q2W). The primary objective was progression-free survival (PFS) of 12 mg/kg-Q2W arm versus placebo from RAINBOW using meta-analysis. Relative to the registered dose, exploratory dosing regimens (EDRs) led to higher ramucirumab serum concentrations in both studies. EDR safety profiles were consistent with previous studies. In JVDB, serious adverse events occurred more frequently in the 8 mg/kg-D1D8-Q3W arm versus the registered dose; 6 mg/kg-QW EDR had a higher incidence of bleeding/hemorrhage. In JVCZ, PFS was improved with the 12 mg/kg plus paclitaxel combination versus placebo in RAINBOW; however, no significant PFS improvement was observed between the 12 mg/kg and 8 mg/kg arms. The lack of a dose/exposure-response relationship in these studies supports the standard dose of ramucirumab 8 mg/kg-Q2W as monotherapy or in combination with paclitaxel as second-line treatment for advanced/metastatic gastric/GEJ adenocarcinoma.
Keywords: angiogenesis; gastric adenocarcinoma; ramucirumab; vascular endothelial growth factor receptor.
Conflict of interest statement
M.A.S. reports grants from Eli Lilly and Company. A.A.U. reports travel and accommodation fees from Amgen, Angelini, Janssen, Merck Sharp & Dohme, Pfizer, and Roche. M.S. reports grants for clinical trials from Eli Lilly and Company, Roche, MSD, Merck, Astra Zeneca, Tesaro, Regeneron Pharmaceuticals, Astellas, Novartis, Bristol Myers Squibb, Pfizer, AbbVie, GlaxoSmithKline, Mylan, Samsung Pharmaceuticals, Bioven, BeiGene, PharmaMar, Clovis, Bayer, Gilead, and Amgen. C.G.-M. reports personal fees from Eli Lilly and Company, Roche, BMS, and Eisai-MSD. H.K. reports research funding from Roche, Pfizer, Novartis, Amgen, Bayer, Genesis, Eli Lilly and Company, MSD, Janssen Pharmaceuticals, and Merck-Serono; consulting/advisory fee from Roche, Novartis, MSD, Genesis, Pfizer, Eli Lilly and Company, LEO Pharma, Amgen, and Merck-Serono; and travel/accommodations fees from Roche, Novartis, Enorasis, and Pfizer. TB reports personal fees and institutional payments for clinical studies from Eli Lilly and Company, Roche, Bristol Myers Squibb, Bayer, Merck, Eisai, Exelixis, AstraZeneca, and Sanofi; personal fees from Astellas, Janssen, Ipsen, and Servier; non-financial support from Bristol Myers Squibb and Ipsen; grants from Servier. P.E. reports honoraria and advisory/consultancy fees from ALX Oncology, Arcus Biosciences, Astellas, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Five Prime Therapeutics, Ideaya Biosciences, Istari Oncology, Legend Biotech, Eli Lilly and Company, Loxo Oncology, Merck, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda Pharmaceutical Company, Turning Point Therapeutics, Xencor, and Zymeworks. L.G. is a former employee of Eli Lilly and Company. R.W., D.F., L.G., and J.M.O. are full-time employees of Eli Lilly and Company. All other authors have declared no conflicts of interest.
Figures


References
-
- CYRAMZA [package insert USA] Eli Lilly and Company; Indianapolis, IN, USA: 2020.
-
- Spratlin J.L., Cohen R.B., Eadens M., Gore L., Camidge D.R., Diab S., Leong S., O’Bryant C., Chow L.Q.M., Serkova N.J., et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully hu-man immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. - DOI - PMC - PubMed
-
- Garon E.B., Ciuleanu T.-E., Arrieta O., Prabhash K., Syrigos K.N., Goksel T., Park K., Gorbunova V., Kowalyszyn R.D., Pikiel J., et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treat-ment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. - DOI - PubMed
-
- Fuchs C.S., Tomasek J., Yong C.J., Dumitru F., Passalacqua R., Goswami C., Safran H., Vieira Dos Santos L., Aprile G., Ferry D.R., et al. REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gas-tro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. - DOI - PubMed
-
- Wilke H., Muro K., Van Cutsem E., Oh S.C., Bodoky G., Shimada Y., Hironaka S., Sugimoto N., Lipatov O., Kim T.-Y., et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previ-ously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

