Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes
- PMID: 35267541
- PMCID: PMC8909265
- DOI: 10.3390/cancers14051233
Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes
Abstract
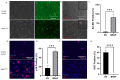
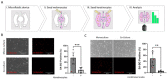
BRAFV600E is the most common mutation driver in melanoma. This mutation is known to cause a brief burst of proliferation followed by growth arrest and senescence, which prevent an uncontrolled cell proliferation. This phenomenon is known as oncogene-induced senescence (OIS) and OIS escape is thought to lead to melanomagenesis. Much attention has been focused on the melanocyte-intrinsic mechanisms that contribute to senescence escape. Additional genetic events such as the loss of tumor suppressor PTEN and/or epigenetic changes that contribute to senescence escape have been described. However, the role of the skin microenvironment-specifically, the role of epidermal keratinocytes-on melanomagenesis is not fully understood. In this study, we employ a microfluidic platform to study the interaction between melanocytes expressing the BRAFV600E mutation as well as keratinocytes and dermal fibroblasts. We demonstrate that keratinocytes suppress senescence-related genes and promote the proliferation of transformed melanocytes. We also show that a keratinocyte-conditioned medium can alter the secretion of both pro- and anti-tumorigenic factors by transformed melanocytes. In addition, we show that melanocytes and keratinocytes from donors of white European and black African ancestry display different crosstalks; i.e., white keratinocytes appear to promote a more pro-tumorigenic phenotype compared with black keratinocytes. These data suggest that keratinocytes exert their influence on melanomagenesis both by suppressing senescence-related genes in melanocytes and by affecting the balance of the melanocyte-secreted factors that favor tumorigenesis.
Keywords: melanoma; microfluidics; senescence; tumor microenvironment.
Conflict of interest statement
D.J.B. holds equity in Bellbrook Labs LLC, Tasso Inc., Salus Discovery LLC, Flambeau Diagnostics LLC, Stacks to the Future LLC, Lynx Biosciences Inc. and Onexio Biosystems.
Figures



References
-
- American Cancer Society Key Statistics for Melanoma Skin Cancer. 2021. [(accessed on 5 September 2021)]. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.....

