Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma
- PMID: 35267563
- PMCID: PMC8909759
- DOI: 10.3390/cancers14051255
Innate and Adaptive Immunopathogeneses in Viral Hepatitis; Crucial Determinants of Hepatocellular Carcinoma
Abstract
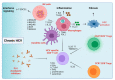
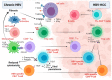
Viral hepatitis B (HBV) and hepatitis C (HCV) infections remain the most common risk factors for the development of hepatocellular carcinoma (HCC), and their heterogeneous distribution influences the global prevalence of this common type of liver cancer. Typical hepatitis infection elicits various immune responses within the liver microenvironment, and viral persistence induces chronic liver inflammation and carcinogenesis. HBV is directly mutagenic but can also cause low-grade liver inflammation characterized by episodes of intermittent high-grade liver inflammation, liver fibrosis, and cirrhosis, which can progress to decompensated liver disease and HCC. Equally, the absence of key innate and adaptive immune responses in chronic HCV infection dampens viral eradication and induces an exhausted and immunosuppressive liver niche that favors HCC development and progression. The objectives of this review are to (i) discuss the epidemiological pattern of HBV and HCV infections, (ii) understand the host immune response to acute and chronic viral hepatitis, and (iii) explore the link between this diseased immune environment and the development and progression of HCC in preclinical models and HCC patients.
Keywords: adaptive immunity; hepatitis B virus; hepatitis C virus; hepatocellular carcinoma; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

