The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays
- PMID: 35267573
- PMCID: PMC8909589
- DOI: 10.3390/cancers14051262
The Effect of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for Cells Irradiated with Grenz Rays
Abstract
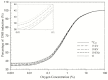
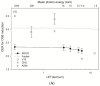
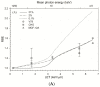
Grenz-ray therapy (GT) is commonly used for dermatological radiotherapy and has a higher linear energy transfer, relative biological effectiveness (RBE) and oxygen enhancement ratio (OER). GT is a treatment option for lentigo maligna and lentigo maligna melanoma. This study aims to calculate the RBE for DNA double-strand break (DSB) induction and cell survival under hypoxic conditions for GT. The yield of DSBs induced by GT is calculated at the aerobic and hypoxic conditions, using a Monte Carlo damage simulation (MCDS) software. The RBE value for cell survival is calculated using the repair-misrepair-fixation (RMF) model. The RBE values for cell survival for cells irradiated by 15 kV, 10 kV and 10 kVp and titanium K-shell X-rays (4.55 kV) relative to 60Co γ-rays are 1.0-1.6 at the aerobic conditions and moderate hypoxia (2% O2), respectively, but increase to 1.2, 1.4 and 1.9 and 2.1 in conditions of severe hypoxia (0.1% O2). The OER values for DSB induction relative to 60Co γ-rays are about constant and ~2.4 for GT, but the OER for cell survival is 2.8-2.0 as photon energy decreases from 15 kV to 4.55 kV. The results indicate that GT results in more DSB induction and allows effective tumor control for superficial and hypoxic tumors.
Keywords: Grenz rays; cell survival; double strand break; hypoxia; oxygen enhancement ratio; relative biological effectiveness.
Conflict of interest statement
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Figures


Similar articles
-
Impact of Hypoxia on Relative Biological Effectiveness and Oxygen Enhancement Ratio for a 62-MeV Therapeutic Proton Beam.Cancers (Basel). 2021 Jun 15;13(12):2997. doi: 10.3390/cancers13122997. Cancers (Basel). 2021. PMID: 34203882 Free PMC article.
-
Monte Carlo Simulation of Double-Strand Break Induction and Conversion after Ultrasoft X-rays Irradiation.Int J Mol Sci. 2021 Oct 28;22(21):11713. doi: 10.3390/ijms222111713. Int J Mol Sci. 2021. PMID: 34769142 Free PMC article.
-
Effects of radiation quality and oxygen on clustered DNA lesions and cell death.Radiat Res. 2011 Nov;176(5):587-602. doi: 10.1667/rr2663.1. Epub 2011 Aug 8. Radiat Res. 2011. PMID: 21823972
-
A comparison of mechanism-inspired models for particle relative biological effectiveness (RBE).Med Phys. 2018 Nov;45(11):e925-e952. doi: 10.1002/mp.13207. Med Phys. 2018. PMID: 30421808 Review.
-
Induction of DNA Damage by Light Ions Relative to 60Co γ-rays.Int J Part Ther. 2018 Summer;5(1):25-39. doi: 10.14338/IJPT-18-00030. Epub 2018 Sep 21. Int J Part Ther. 2018. PMID: 31773018 Free PMC article. Review.
Cited by
-
The Importance of the Tumor Microenvironment to Understand Tumor Origin, Evolution, and Treatment Response.Cancers (Basel). 2022 Apr 14;14(8):1983. doi: 10.3390/cancers14081983. Cancers (Basel). 2022. PMID: 35454888 Free PMC article.
-
Effect of Hematocrit Injury on the Survival Rate of Advanced Malignant Tumors and Its Clinical Significance.Comput Math Methods Med. 2022 Jun 15;2022:4968754. doi: 10.1155/2022/4968754. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Dec 6;2023:9784208. doi: 10.1155/2023/9784208. PMID: 35756408 Free PMC article. Retracted.
-
Oxygen Effect on 0-30 eV Electron Damage to DNA Under Different Hydration Levels: Base and Clustered Lesions, Strand Breaks and Crosslinks.Molecules. 2024 Dec 21;29(24):6033. doi: 10.3390/molecules29246033. Molecules. 2024. PMID: 39770123 Free PMC article.
References
-
- Panizzon R.G. Grenz rays: An alternative treatment for superficial skin cancers in elderly patients. Aging Health. 2009;5:495–496. doi: 10.2217/ahe.09.46. - DOI
-
- Panizzon R.G., Seegenschmiedt M.H. Radiation Treatment and Radiation Reactions in Dermatology. Springer; Berlin/Heidelberg, Germany: 2015.
-
- Hanlon A. A Practical Guide to Skin Cancer. Springer; Cham, Switzerland: 2018.
Grants and funding
- CSMU-INT-110-12/Chung Shan Medical University, Taichung, Taiwan
- CMRPD1H0473/Linkou-Chang Gung Memorial Hospital, Taoyuan, Taiwan
- CMRPD1J0322/Linkou-Chang Gung Memorial Hospital, Taoyuan, Taiwan
- MOST 109-2314-B-182-078-MY3/Ministry of Sciences and Technology, Taiwan
- MOST 110-2628-B-182-008/Ministry of Sciences and Technology, Taiwan
LinkOut - more resources
Full Text Sources
Miscellaneous

