3D Printed Scaffold Based on Type I Collagen/PLGA_TGF-β1 Nanoparticles Mimicking the Growth Factor Footprint of Human Bone Tissue
- PMID: 35267680
- PMCID: PMC8912467
- DOI: 10.3390/polym14050857
3D Printed Scaffold Based on Type I Collagen/PLGA_TGF-β1 Nanoparticles Mimicking the Growth Factor Footprint of Human Bone Tissue
Abstract
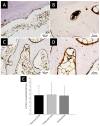
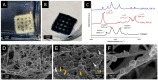
In bone regenerative strategies, the controlled release of growth factors is one of the main aspects for successful tissue regeneration. Recent trends in the drug delivery field increased the interest in the development of biodegradable systems able to protect and transport active agents. In the present study, we designed degradable poly(lactic-co-glycolic)acid (PLGA) nanocarriers suitable for the release of Transforming Growth Factor-beta 1 (TGF-β1), a key molecule in the management of bone cells behaviour. Spherical TGF-β1-containing PLGA (PLGA_TGF-β1) nanoparticles (ca.250 nm) exhibiting high encapsulation efficiency (ca.64%) were successfully synthesized. The TGF-β1 nanocarriers were subsequently combined with type I collagen for the fabrication of nanostructured 3D printed scaffolds able to mimic the TGF-β1 presence in the human bone extracellular matrix (ECM). The homogeneous hybrid formulation underwent a comprehensive rheological characterisation in view of 3D printing. The 3D printed collagen-based scaffolds (10 mm × 10 mm × 1 mm) successfully mimicked the TGF-β1 presence in human bone ECM as assessed by immunohistochemical TGF-β1 staining, covering ca.3.4% of the whole scaffold area. Moreover, the collagenous matrix was able to reduce the initial burst release observed in the first 24 h from about 38% for the PLGA_TGF-β1 alone to 14.5%, proving that the nanocarriers incorporation into collagen allows achieving sustained release kinetics.
Keywords: 3D printed scaffolds; TGF-β1; bone; drug delivery; polymeric nanoparticles; tissue regeneration; type I collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Moreira C.A., Dempster D.W., Baron R., Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., et al. Endotext [Internet] MDText.com, Inc.; South Dartmouth, MA, USA: 2000. Anatomy and ultrastructure of bone-histogenesis, growth and remodeling.
-
- Bismar H., Kloppinger T., Kloppinger K., Schuster E.M., Balbach S., Diel I., Ziegler R., Pfeilschifter J. Transforming growth factor (TGF-) levels in the conditioned media of human bone cells: Relationship to donor age, bone volume, and concentration of TGF-in human bone matrix in vivo. Bone. 1999;24:565–569. doi: 10.1016/S8756-3282(99)00082-4. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

