Chicory: Understanding the Effects and Effectors of This Functional Food
- PMID: 35267932
- PMCID: PMC8912540
- DOI: 10.3390/nu14050957
Chicory: Understanding the Effects and Effectors of This Functional Food
Abstract
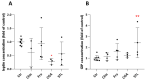
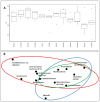
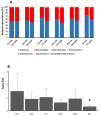
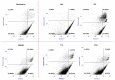
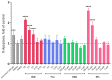
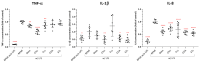
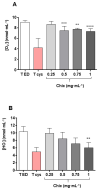
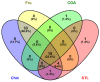
Industrial chicory has been the subject of numerous studies, most of which provide clinical observations on its health effects. Whether it is the roasted root, the flour obtained from the roots or the different classes of molecules that enter into the composition of this plant, understanding the molecular mechanisms of action on the human organism remains incomplete. In this study, we were interested in three molecules or classes of molecules present in chicory root: fructose, chlorogenic acids, and sesquiterpene lactones. We conducted experiments on the murine model and performed a nutrigenomic analysis, a metabolic hormone assay and a gut microbiota analysis, associated with in vitro observations for different responses. We have highlighted a large number of effects of all these classes of molecules that suggest a pro-apoptotic activity, an anti-inflammatory, antimicrobial, antioxidant, hypolipidemic and hypoglycemic effect and also an important role in appetite regulation. A significant prebiotic activity was also identified. Fructose seems to be the most involved in these activities, contributing to approximately 83% of recorded responses, but the other classes of tested molecules have shown a specific role for these different effects, with an estimated contribution of 23-24%.
Keywords: chicory; gut microbiota; hormone assay; in vitro apoptosis; in vitro pro-inflammatory cytokines; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Perović J., Tumbas Šaponjac V., Kojić J., Krulj J., Moreno D.A., García-Viguera C., Bodroža-Solarov M., Ilić N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021;336:127676. doi: 10.1016/j.foodchem.2020.127676. - DOI - PubMed
-
- Pouille C.L., Jegou D., Dugardin C., Cudennec B., Ravallec R., Hance P., Rambaud C., Hilbert J.L., Lucau-Danila A. Chicory root flour—A functional food with potential multiple health benefits evaluated in a mice model. J. Funct. Foods. 2020;74:104174. doi: 10.1016/j.jff.2020.104174. - DOI
-
- Reimer R.A., Soto-Vaca A., Nicolucci A.C., Mayengbam S., Park H., Madsen K.L., Menon R., Vaughan E.E. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020;111:1286–1296. doi: 10.1093/ajcn/nqaa074. - DOI - PubMed
-
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) Frutalose®, a mixture of fructans obtained from enzymatic hydrolysis of chicory inulin, and normal defecation: Evaluation of a health claim pursuant to Article 13 of Regulation (EC) No 1924/2006. EFSA J. 2021;19:e06775. doi: 10.2903/j.efsa.2021.6775. - DOI - PMC - PubMed
-
- Ferrare K., Bidel L.P., Awwad A., Poucheret P., Cazals G., Lazennec F., Azay-Milhau J., Tournier M., Lajoix A.D., Tousch D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018;215:241–248. doi: 10.1016/j.jep.2017.12.035. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

