The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis
- PMID: 35268009
- PMCID: PMC8912513
- DOI: 10.3390/nu14051036
The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis
Abstract
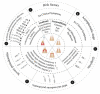
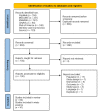
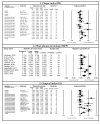
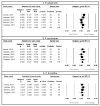
(1) Background: Periodontal diseases are a global health concern. They are multi-stage, progressive inflammatory diseases triggered by the inflammation of the gums in response to periodontopathogens and may lead to the destruction of tooth-supporting structures, tooth loss, and systemic health problems. This systematic review and meta-analysis evaluated the effects of probiotic supplementation on the prevention and treatment of periodontal disease based on the assessment of clinical, microbiological, and immunological outcomes. (2) Methods: This study was registered under PROSPERO (CRD42021249120). Six databases were searched: PubMed, MEDLINE, EMBASE, CINAHL, Web of Science, and Dentistry and Oral Science Source. The meta-analysis assessed the effects of probiotic supplementation on the prevention and treatment of periodontal diseases and reported them using Hedge's g standardized mean difference (SMD). (3) Results: Of the 1883 articles initially identified, 64 randomized clinical trials were included in this study. The results of this meta-analysis indicated statistically significant improvements after probiotic supplementation in the majority of the clinical outcomes in periodontal disease patients, including the plaque index (SMD = 0.557, 95% CI: 0.228, 0.885), gingival index, SMD = 0.920, 95% CI: 0.426, 1.414), probing pocket depth (SMD = 0.578, 95% CI: 0.365, 0.790), clinical attachment level (SMD = 0.413, 95% CI: 0.262, 0.563), bleeding on probing (SMD = 0.841, 95% CI: 0.479, 1.20), gingival crevicular fluid volume (SMD = 0.568, 95% CI: 0.235, 0.902), reduction in the subgingival periodontopathogen count of P. gingivalis (SMD = 0.402, 95% CI: 0.120, 0.685), F. nucleatum (SMD = 0.392, 95% CI: 0.127, 0.658), and T. forsythia (SMD = 0.341, 95% CI: 0.050, 0.633), and immunological markers MMP-8 (SMD = 0.819, 95% CI: 0.417, 1.221) and IL-6 (SMD = 0.361, 95% CI: 0.079, 0.644). (4) Conclusions: The results of this study suggest that probiotic supplementation improves clinical parameters, and reduces the periodontopathogen load and pro-inflammatory markers in periodontal disease patients. However, we were unable to assess the preventive role of probiotic supplementation due to the paucity of studies. Further clinical studies are needed to determine the efficacy of probiotic supplementation in the prevention of periodontal diseases.
Keywords: clinical parameters; gingivitis; oral health; periodontal disease; periodontitis; prevention; probiotic; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Manual versus powered toothbrushing for oral health.Cochrane Database Syst Rev. 2003;(1):CD002281. doi: 10.1002/14651858.CD002281. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2005 Apr 18;(2):CD002281. doi: 10.1002/14651858.CD002281.pub2. PMID: 12535436 Updated.
-
Guided tissue regeneration for periodontal infra-bony defects.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD001724. doi: 10.1002/14651858.CD001724.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD001724. doi: 10.1002/14651858.CD001724.pub3. PMID: 16625546 Updated.
-
Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression.Nutrients. 2022 Jan 30;14(3):622. doi: 10.3390/nu14030622. Nutrients. 2022. PMID: 35276980 Free PMC article.
-
Treatment of periodontitis for glycaemic control in people with diabetes mellitus.Cochrane Database Syst Rev. 2022 Apr 14;4(4):CD004714. doi: 10.1002/14651858.CD004714.pub4. Cochrane Database Syst Rev. 2022. PMID: 35420698 Free PMC article.
-
Music interventions for improving psychological and physical outcomes in people with cancer.Cochrane Database Syst Rev. 2021 Oct 12;10(10):CD006911. doi: 10.1002/14651858.CD006911.pub4. Cochrane Database Syst Rev. 2021. PMID: 34637527 Free PMC article.
Cited by
-
Sponsorship Bias in Clinical Trials in the Dental Application of Probiotics: A Meta-Epidemiological Study.Nutrients. 2022 Aug 19;14(16):3409. doi: 10.3390/nu14163409. Nutrients. 2022. PMID: 36014917 Free PMC article.
-
RANKL, OPG, and CTS-K Release in Bone Response to Immediate Nonfunctional Loading of a Single Implant in Mandibular Molar Sites During Osseointegration Establishment.Clin Exp Dent Res. 2025 Aug;11(4):e70193. doi: 10.1002/cre2.70193. Clin Exp Dent Res. 2025. PMID: 40792611 Free PMC article. Clinical Trial.
-
Association between different dietary patterns and eating disorders and periodontal diseases.Front Oral Health. 2023 Mar 22;4:1152031. doi: 10.3389/froh.2023.1152031. eCollection 2023. Front Oral Health. 2023. PMID: 37035252 Free PMC article. Review.
-
Microbiological and clinical effects of probiotic-related Zeger therapy on gingival health: a randomized controlled clinical trial.BMC Oral Health. 2024 Sep 14;24(1):1086. doi: 10.1186/s12903-024-04846-x. BMC Oral Health. 2024. PMID: 39277730 Free PMC article. Clinical Trial.
-
The role of probiotics and dietary interventions in the treatment of periodontitis: a pilot randomized controlled clinical trial.BMC Oral Health. 2025 Jul 31;25(1):1287. doi: 10.1186/s12903-025-06510-4. BMC Oral Health. 2025. PMID: 40745652 Free PMC article. Clinical Trial.
References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- Gasner N.S., Schure R.S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2021. Periodontal Disease.
Publication types
MeSH terms
Substances
Grants and funding
- Patient-oriented Research Leadership Grant/Saskatchewan Centre for Patient Oriented Research (SCPOR)
- Patient-oriented Research Leadership Grant/Saskatchewan Health Research Foundation
- SCPOR/Saskatchewan Centre for Patient Oriented Research Graduate and Postdoctoral Fellowships
- College of Medicine CoMGRAD Graduate Student Fellowship/University of Saskatchewan
- Department of Biochemistry, Microbiology and Immunology Devolved Scholarship/University of Saskatchewan
- Saskatchewan Innovation and Opportunity Scholarship
- Alpha Omega Foundation OF Canada
- College of Medicine start-up funds/University of Saskatchewan
- College of Dentistry start-up funds/University of Saskatchewan
- Centennial Enhancement Chair in One Health Research/University of Saskatchewan
- College of Pharmacy and Nutrition start-up funds/University of Saskatchewan
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

