Relevance of Aquaporins for Gamete Function and Cryopreservation
- PMID: 35268142
- PMCID: PMC8909058
- DOI: 10.3390/ani12050573
Relevance of Aquaporins for Gamete Function and Cryopreservation
Abstract
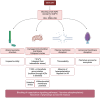
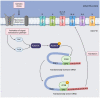
The interaction between cells and the extracellular medium is of great importance, and drastic changes in extracellular solute concentrations drive water movement across the plasma membrane. Aquaporins (AQPs) are a family of transmembrane channels that allow the transport of water and small solutes across cell membranes. Different members of this family have been identified in gametes. In sperm, they are relevant to osmoadaptation after entering the female reproductive tract, which is crucial for sperm motility activation and capacitation and, thus, for their fertilizing ability. In addition, they are relevant during the cryopreservation process, since some members of this family are also permeable to glycerol, one of the most frequently used cryoprotective agents in livestock. Regarding oocytes, AQPs are very important in their maturation but also during cryopreservation. Further research to define the exact sets of AQPs that are present in oocytes from different species is needed, since the available literature envisages certain AQPs and their roles but does not provide complete information on the whole set of AQPs. This is of considerable importance because, in sperm, specific AQPs are known to compensate the role of non-functional members.
Keywords: cryopreservation; mammals; oocyte; physiology; sperm; water channels.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Aquaporins Are Essential to Maintain Motility and Membrane Lipid Architecture During Mammalian Sperm Capacitation.Front Cell Dev Biol. 2021 Sep 1;9:656438. doi: 10.3389/fcell.2021.656438. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34540822 Free PMC article.
-
Aquaporins and Animal Gamete Cryopreservation: Advances and Future Challenges.Animals (Basel). 2022 Feb 2;12(3):359. doi: 10.3390/ani12030359. Animals (Basel). 2022. PMID: 35158682 Free PMC article. Review.
-
Structure, function, and localization of aquaporins: their possible implications on gamete cryopreservation.Genet Mol Res. 2013 Dec 13;12(4):6718-32. doi: 10.4238/2013.December.13.5. Genet Mol Res. 2013. PMID: 24391013 Review.
-
Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology.Reprod Domest Anim. 2017 Oct;52 Suppl 4:12-27. doi: 10.1111/rda.13082. Reprod Domest Anim. 2017. PMID: 29052330 Review.
-
Aquaglyceroporins but not orthodox aquaporins are involved in the cryotolerance of pig spermatozoa.J Anim Sci Biotechnol. 2019 Oct 14;10:77. doi: 10.1186/s40104-019-0388-8. eCollection 2019. J Anim Sci Biotechnol. 2019. PMID: 31636902 Free PMC article.
Cited by
-
Glycerol-Free Equilibration with the Addition of Glycerol Shortly before the Freezing Procedure: A Perspective Strategy for Cryopreservation of Wallachian Ram Sperm.Animals (Basel). 2023 Mar 29;13(7):1200. doi: 10.3390/ani13071200. Animals (Basel). 2023. PMID: 37048456 Free PMC article.
-
From infection to infertility: a review of the role of human papillomavirus-induced oxidative stress on reproductive health and infertility.Eur J Med Res. 2025 Apr 28;30(1):339. doi: 10.1186/s40001-025-02605-4. Eur J Med Res. 2025. PMID: 40296084 Free PMC article. Review.
-
Oviducal gland transcriptomics of Octopus maya through physiological stages and the negative effects of temperature on fertilization.PeerJ. 2022 Mar 30;10:e12895. doi: 10.7717/peerj.12895. eCollection 2022. PeerJ. 2022. PMID: 35378931 Free PMC article.
-
Important functions and molecular mechanisms of aquaporins family on respiratory diseases: potential translational values.J Cancer. 2024 Oct 7;15(18):6073-6085. doi: 10.7150/jca.98829. eCollection 2024. J Cancer. 2024. PMID: 39440058 Free PMC article. Review.
-
Signaling Roleplay between Ion Channels during Mammalian Sperm Capacitation.Biomedicines. 2023 Sep 12;11(9):2519. doi: 10.3390/biomedicines11092519. Biomedicines. 2023. PMID: 37760960 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

