One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice
- PMID: 35268255
- PMCID: PMC8911125
- DOI: 10.3390/jcm11051164
One-Year Outcomes and Trends over Two Eras of Transcatheter Aortic Valve Implantation in Real-World Practice
Abstract
Background: Data reflecting the benefit of procedural improvements in real-world transcatheter aortic valve implantation (TAVI) practice are sparse.
Aims: To compare outcomes and trends of two TAVI eras from real Italian practice.
Methods: A total of 1811 and 2939 TAVI patients enrolled in the national, prospective OBSERVANT and OBSERVANT II studies in 2010-2012 and 2016-2018, respectively, were compared in a cohort study. Outcomes were adjusted using inverse propensity of treatment weighting and propensity score matching.
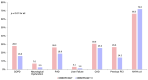
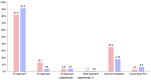
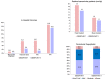
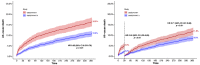
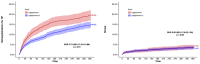
Results: The median age (83.0 (79.0-86.0) vs. 83.0 (79.0-86.0)) and EuroSCORE II (5.2 (3.2-7.7) vs. 5.1 (3.1-8.1)) of OBSERVANT and OBSERVANT II patients were similar. At 1 year, patients of the OBSERVANT II study had a significantly lower risk of all-cause death (10.6% vs. 16.3%, Hazard Ratio (HR) 0.63 (95% Confidence Interval (CI) 0.52-0.76)) and rehospitalization for heart failure (HF) (14.3% vs. 19.5%, Sub-distribution HR 0.71 (95%CI 0.60-0.84)), whereas rates of stroke (3.1% vs. 3.6%) and permanent pacemaker implantation (PPI) (16.6% vs. 18.0%) were comparable between study groups.
Conclusions: Age and risk profile among patients undergoing TAVI in Italy remained substantially unchanged between the 2010-2012 and 2016-2018 time periods. After adjustment, patients undergoing TAVI in the most recent era had lower risk of all-cause death and rehospitalization for HF at 1 year, whereas rates of stroke and PPI did not differ significantly.
Keywords: OBSERVANT; outcomes; transcatheter aortic valve implantation; trends.
Conflict of interest statement
Corrado Tamburino is a consultant for Medtronic; Marco Barbanti is a consultant for Edwards LifeSciences, Medtronic and Boston Scientifics; Giuseppe Tarantini reports honoraria for lectures/consulting from Edwards LifeSciences, Medtronic and Boston Scientifics and GADA. All other authors have no conflict of interest to declare.
Figures

References
-
- Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., Kapadia S.R., Malaisrie S.C., Cohen D.J., Pibarot P., et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. - DOI - PubMed
-
- Barbanti M., Buccheri S., Rodés-Cabau J., Gulino S., Généreux P., Pilato G., Dvir D., Picci A., Costa G., Tamburino C., et al. Transcatheter aortic valve replacement with new-generation devices: A systematic review and meta-analysis. Int. J. Cardiol. 2017;245:83–89. doi: 10.1016/j.ijcard.2017.07.083. - DOI - PubMed
-
- Auffret V., Lefevre T., Van Belle E., Eltchaninoff H., Iung B., Koning R., Motreff P., Leprince P., Verhoye J.P., Manigold T., et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017;70:42–55. doi: 10.1016/j.jacc.2017.04.053. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

