The Impact of SARS-CoV-2 Primary Vaccination in a Cohort of Patients Hospitalized for Acute COVID-19 during Delta Variant Predominance
- PMID: 35268282
- PMCID: PMC8911274
- DOI: 10.3390/jcm11051191
The Impact of SARS-CoV-2 Primary Vaccination in a Cohort of Patients Hospitalized for Acute COVID-19 during Delta Variant Predominance
Abstract
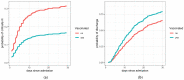
Vaccine breakthrough SARS-CoV-2 infections necessitating hospitalization have emerged as a relevant problem with longer time interval since vaccination and the predominance of the Delta variant. The aim of this study was to evaluate the association between primary vaccination with four SARS-CoV-2 vaccines authorized for use in the European Union-BNT162b2, ChAdOx-1S, mRNA-1273 or Ad.26.COV2.S-and progression to critically severe disease (mechanical ventilation or death) and duration of hospitalization among adult patients with PCR-confirmed acute COVID-19 hospitalized during the Delta variant predominance (October-November 2021) in Slovenia. Among the 529 enrolled patients hospitalized with COVID-19 (median age, 65 years; 58.2% men), 175 (33.1%) were fully vaccinated at the time of symptom onset. Compared with 345 unvaccinated patients, fully vaccinated patients with breakthrough infections were older, more often immunocompromised, and had higher Charlson comorbidity index scores. After adjusting for sex, age, and comorbidities, fully vaccinated patients had lower odds for progressing to critically severe disease and were discharged from the hospital earlier than unvaccinated patients. Vaccination against SARS-CoV-2 remains an extremely effective intervention to alleviate morbidity and mortality in COVID-19 patients.
Keywords: COVID-19 outcome; COVID-19 vaccine; vaccine breakthrough.
Conflict of interest statement
D.S. received support to speak at educational events sponsored by MSD, Sanofi, and Medison Pharma. The other authors report no potential conflict.
Figures
Similar articles
-
Association between COVID-19 Primary Vaccination and Severe Disease Caused by SARS-CoV-2 Delta Variant among Hospitalized Patients: A Belgian Retrospective Cohort Study.Vaccines (Basel). 2022 Dec 21;11(1):14. doi: 10.3390/vaccines11010014. Vaccines (Basel). 2022. PMID: 36679859 Free PMC article.
-
SARS-CoV-2 Vaccination and Clinical Presentation of COVID-19 in Patients Hospitalized during the Delta- and Omicron-Predominant Periods.J Clin Med. 2023 Jan 26;12(3):961. doi: 10.3390/jcm12030961. J Clin Med. 2023. PMID: 36769609 Free PMC article.
-
Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance - One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022.MMWR Morb Mortal Wkly Rep. 2022 Feb 11;71(6):217-223. doi: 10.15585/mmwr.mm7106e2. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35143466 Free PMC article.
-
SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status - Los Angeles County, California, May 1-July 25, 2021.MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1170-1176. doi: 10.15585/mmwr.mm7034e5. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34437525 Free PMC article.
-
Characteristics of COVID-19 Breakthrough Infections among Vaccinated Individuals and Associated Risk Factors: A Systematic Review.Trop Med Infect Dis. 2022 May 22;7(5):81. doi: 10.3390/tropicalmed7050081. Trop Med Infect Dis. 2022. PMID: 35622708 Free PMC article. Review.
Cited by
-
How Does Vaccination against SARS-CoV-2 Affect Hospitalized Patients with COVID-19?J Clin Med. 2022 Jul 5;11(13):3905. doi: 10.3390/jcm11133905. J Clin Med. 2022. PMID: 35807189 Free PMC article.
-
Epidemiological and clinical characteristics of vaccinated COVID-19 patients: A meta-analysis and systematic review.Int J Immunopathol Pharmacol. 2022 Jan-Dec;36:3946320221141802. doi: 10.1177/03946320221141802. Int J Immunopathol Pharmacol. 2022. PMID: 36412572 Free PMC article.
-
Association between COVID-19 Primary Vaccination and Severe Disease Caused by SARS-CoV-2 Delta Variant among Hospitalized Patients: A Belgian Retrospective Cohort Study.Vaccines (Basel). 2022 Dec 21;11(1):14. doi: 10.3390/vaccines11010014. Vaccines (Basel). 2022. PMID: 36679859 Free PMC article.
-
Global trends in COVID-19 Alzheimer's related research: a bibliometric analysis.Front Neurol. 2023 Jun 5;14:1193768. doi: 10.3389/fneur.2023.1193768. eCollection 2023. Front Neurol. 2023. PMID: 37342784 Free PMC article.
-
The effect of COVID-19 vaccination status on all-cause mortality in patients hospitalised with COVID-19 in Hungary during the delta wave of the pandemic.Geroscience. 2024 Apr;46(2):1881-1894. doi: 10.1007/s11357-023-00931-1. Epub 2023 Sep 27. Geroscience. 2024. PMID: 37755581 Free PMC article.
References
-
- Herrington D.M., Sanders J.H., Wierzba T.F., Alexander-Miller M., Espeland M., Bertoni A.G., Mathews A., Seals A.L., Munawar I., Runyon M.S., et al. Duration of SARS-CoV-2 sero-positivity in a large longitudinal sero-surveillance cohort: The COVID-19 Community Research Partnership. BMC Infect. Dis. 2021;21:889. - PMC - PubMed
-
- Rijkers G., Murk J.-L., Wintermans B., Van Looy B., Berge M.V.D., Veenemans J., Stohr J., Reusken C., Van Der Pol P., Reimerink J. Differences in Antibody Kinetics and Functionality Between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. J. Infect. Dis. 2020;222:1265–1269. doi: 10.1093/infdis/jiaa463. - DOI - PMC - PubMed
-
- Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

