Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement
- PMID: 35268436
- PMCID: PMC8911237
- DOI: 10.3390/jcm11051338
Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement
Abstract
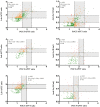
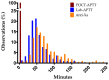
Continuous intravenous unfractionated heparin (UFH) is administered routinely in the intensive care unit (ICU) for the anticoagulation of patients, and monitoring is performed by the activated partial thromboplastin time (APTT) or anti-Xa activity. However, these strategies are associated with potentially large time intervals before dose adjustments, which could be detrimental to the patient. The aim of the study was to compare a point-of-care (POCT) version of the APTT to (i) laboratory-based APTT and (ii) measurements of anti-Xa activity in terms of correlation, agreement and turnaround time (TAT). Thirty-five ICU patients requiring UFH therapy were prospectively included and followed longitudinally for a maximum duration of 15 days. UFH was administered according to a local adaptation of Raschke and Amanzadeh’s aPTT nomograms. Simultaneous measurements of POCT-APTT (CoaguCheck® aPTT Test, Roche Diagnostics) on a drop of fresh whole blood, laboratory-based APTT (C.K. Prest®, Stago) and anti-Xa activity (STA®Liquid anti-Xa, Stago) were systematically performed two to six times a day. Antithrombin, C-reactive protein, fibrinogen, factor VIII and lupus anticoagulant were measured. The time tracking of sampling and analysis was recorded. The overall correlation between POCT-APTT and laboratory APTT (n = 795 pairs) was strongly positive (rs = 0.77, p < 0.0001), and between POCT-APTT and anti-Xa activity (n = 729 pairs) was weakly positive (rs = 0.46, p < 0.0001). Inter-method agreement (Cohen’s kappa (k)) between POCT and laboratory APTT was 0.27, and between POCT and anti-Xa activity was 0.30. The median TATs from sample collection to the lab delivery of results for lab-APTT and anti-Xa were 50.9 min (interquartile range (IQR), 38.4−69.1) and 66.3 min (IQR, 49.0−91.8), respectively, while the POCT delivered results in less than 5 min (p < 0.0001). Although the use of the POCT-APTT device significantly reduced the time to results, the results obtained were poorly consistent with those obtained by lab-APTT or anti-Xa activity, and therefore it should not be used with the nomograms developed for lab-APTT.
Keywords: APTT; POCT; anti-Xa; heparin; monitoring; unfractionated heparin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The Extracorporeal Life Support Organization (ELSO) Extracorporeal Life Support Organization (ELSO) General Guidelines for All ECLS Cases. [(accessed on 14 January 2022)]. Available online: https://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%...
-
- Whitman-Purves E., Coons J.C., Miller T., DiNella J.V., Althouse A., Schmidhofer M., Smith R.E. Performance of Anti-Factor Xa Versus Activated Partial Thromboplastin Time for Heparin Monitoring Using Multiple Nomograms. Clin. Appl. Thromb. Hemost. 2018;24:310–316. doi: 10.1177/1076029617741363. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

