Early Access to Medicines: Use of Multicriteria Decision Analysis (MCDA) as a Decision Tool in Catalonia (Spain)
- PMID: 35268443
- PMCID: PMC8910942
- DOI: 10.3390/jcm11051353
Early Access to Medicines: Use of Multicriteria Decision Analysis (MCDA) as a Decision Tool in Catalonia (Spain)
Abstract
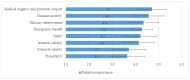
Early access to medicines allows the prescription of a medicine before it is available in the public formulary to patients with severe or rare diseases with high unmet needs who have no authorised therapeutic alternatives available. In this context, consistent decision making is difficult, and a systematic assessment procedure could be useful to tackle complex situations and guarantee the equity of medicines' access. A multidisciplinary panel (MP) conducted four workshops to develop an early access framework based on a reflective multiple criteria decision analysis (MCDA). A set of 12 criteria was agreed: eight quantitative (severity of disease, urgency, efficacy, safety, internal and external validity, therapeutic benefit and plausibility) and four qualitative (therapeutic alternative, existence of precedents, management impact and costs). Quantitative criteria were weighted using a five-point scale. The relative importance of quantitative criteria had mean weights from 4.7 to 3.6, showing its relevance in the decisions. The framework was tested using two case studies, and reliability was assessed by re-test. The re-test revealed no statistical differences, indicating the consistency and replicability of the framework developed. MCDA may help to structure discussions for heterogeneous treatment requests, providing predictability and robustness in decision making involving sensitive and complex situations.
Keywords: MCDA; assessment; drug policy; early access; multicriteria decision analysis; pharmaceuticals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Implementing reflective multicriteria decision analysis (MCDA) to assess orphan drugs value in the Catalan Health Service (CatSalut).Orphanet J Rare Dis. 2019 Jun 27;14(1):157. doi: 10.1186/s13023-019-1121-6. Orphanet J Rare Dis. 2019. PMID: 31248421 Free PMC article.
-
Multicriteria decision analysis (MCDA) for health technology assessment: the Queensland Health experience.Aust Health Rev. 2019 Oct;43(5):591-599. doi: 10.1071/AH18042. Aust Health Rev. 2019. PMID: 30205873
-
Bridging health technology assessment (HTA) and efficient health care decision making with multicriteria decision analysis (MCDA): applying the EVIDEM framework to medicines appraisal.Med Decis Making. 2012 Mar-Apr;32(2):376-88. doi: 10.1177/0272989X11416870. Epub 2011 Oct 10. Med Decis Making. 2012. PMID: 21987539
-
Identifying key unmet needs and value drivers in the treatment of focal-onset seizures (FOS) in patients with drug-resistant epilepsy (DRE) in Spain through Multi-Criteria Decision Analysis (MCDA).Epilepsy Behav. 2021 Sep;122:108222. doi: 10.1016/j.yebeh.2021.108222. Epub 2021 Aug 6. Epilepsy Behav. 2021. PMID: 34371462
-
A multicriteria decision analysis (MCDA) tool to purchase implantable medical devices in Egypt.BMC Med Inform Decis Mak. 2022 Nov 9;22(1):289. doi: 10.1186/s12911-022-02025-y. BMC Med Inform Decis Mak. 2022. PMID: 36352382 Free PMC article.
Cited by
-
Determining value in the treatment of activated PI3Kδ syndrome in Spain: a multicriteria decision analysis from the perspective of key stakeholders.Glob Reg Health Technol Assess. 2024 May 22;11:124-130. doi: 10.33393/grhta.2024.3041. eCollection 2024 Jan-Dec. Glob Reg Health Technol Assess. 2024. PMID: 38784663 Free PMC article.
-
Using multi-criteria decision analysis to describe stakeholder preferences for new quality improvement initiatives that could optimise prescribing in England.Front Health Serv. 2023 Jun 20;3:1155523. doi: 10.3389/frhs.2023.1155523. eCollection 2023. Front Health Serv. 2023. PMID: 37409178 Free PMC article.
-
Value contribution of leniolisib in the Treatment of Activated PI3Kδ syndrome (APDS) in Spain using Multi-Criteria Decision Analysis (MCDA).Glob Reg Health Technol Assess. 2025 Jan 27;12:9-15. doi: 10.33393/grhta.2025.3199. eCollection 2025 Jan-Dec. Glob Reg Health Technol Assess. 2025. PMID: 39882388 Free PMC article.
-
Equity and capacity to benefit from early access to medicines schemes.Int J Equity Health. 2025 Apr 10;24(1):100. doi: 10.1186/s12939-025-02416-3. Int J Equity Health. 2025. PMID: 40211343 Free PMC article. Review.
References
-
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. Springer; Berlin/Heidelberg, Germany: 2001. European Parliament. - DOI
-
- World Health Organization—WHO . Medicines Reimbursement Policies in Europe. Regional Office for Europe; Geneva, Switzerland: 2018.

