Detection of Vulnerable Coronary Plaques Using Invasive and Non-Invasive Imaging Modalities
- PMID: 35268451
- PMCID: PMC8911129
- DOI: 10.3390/jcm11051361
Detection of Vulnerable Coronary Plaques Using Invasive and Non-Invasive Imaging Modalities
Abstract
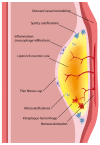
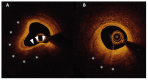
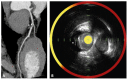
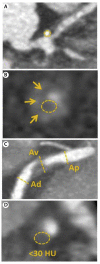
Acute coronary syndrome (ACS) mostly arises from so-called vulnerable coronary plaques, particularly prone for rupture. Vulnerable plaques comprise a specific type of plaque, called the thin-cap fibroatheroma (TFCA). A TCFA is characterized by a large lipid-rich necrotic core, a thin fibrous cap, inflammation, neovascularization, intraplaque hemorrhage, microcalcifications or spotty calcifications, and positive remodeling. Vulnerable plaques are often not visible during coronary angiography. However, different plaque features can be visualized with the use of intracoronary imaging techniques, such as intravascular ultrasound (IVUS), potentially with the addition of near-infrared spectroscopy (NIRS), or optical coherence tomography (OCT). Non-invasive imaging techniques, such as computed tomography coronary angiography (CTCA), cardiovascular magnetic resonance (CMR) imaging, and nuclear imaging, can be used as an alternative for these invasive imaging techniques. These invasive and non-invasive imaging modalities can be implemented for screening to guide primary or secondary prevention therapies, leading to a more patient-tailored diagnostic and treatment strategy. Systemic pharmaceutical treatment with lipid-lowering or anti-inflammatory medication leads to plaque stabilization and reduction of cardiovascular events. Additionally, ongoing studies are investigating whether modification of vulnerable plaque features with local invasive treatment options leads to plaque stabilization and subsequent cardiovascular risk reduction.
Keywords: intracoronary imaging; non-invasive imaging; thin-cap fibroatheroma; vulnerable plaque.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


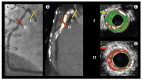
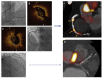
References
-
- Kolansky D.M. Acute coronary syndromes: Morbidity, mortality, and pharmacoeconomic burden. Am. J. Manag. Care. 2009;15:S36–S41. - PubMed
-
- Brown B.G., Gallery C.A., Badger R.S., Kennedy J.W., Mathey D., Bolson E.L., Dodge H.T. Incomplete lysis of thrombus in the moderate underlying atherosclerotic lesion during intracoronary infusion of streptokinase for acute myocardial infarction: Quantitative angiographic observations. Circulation. 1986;73:653–661. doi: 10.1161/01.CIR.73.4.653. - DOI - PubMed
-
- Little W.C., Constantinescu M., Applegate R.J., Kutcher M.A., Burrows M.T., Kahl F.R., Santamore W.P. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.CIR.78.5.1157. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

