Automated Phasor Segmentation of Fluorescence Lifetime Imaging Data for Discriminating Pigments and Binders Used in Artworks
- PMID: 35268575
- PMCID: PMC8911548
- DOI: 10.3390/molecules27051475
Automated Phasor Segmentation of Fluorescence Lifetime Imaging Data for Discriminating Pigments and Binders Used in Artworks
Abstract
The non-invasive analysis of fluorescence from binders and pigments employed in mixtures in artworks is a major challenge in cultural heritage science due to the broad overlapping emission of different fluorescent species causing difficulties in the data interpretation. To improve the specificity of fluorescence measurements, we went beyond steady-state fluorescence measurements by resolving the fluorescence decay dynamics of the emitting species through time-resolved fluorescence imaging (TRFI). In particular, we acquired the fluorescence decay features of different pigments and binders using a portable and compact fibre-based imaging setup. Fluorescence time-resolved data were analysed using the phasor method followed by a Gaussian mixture model (GMM) to automatically identify the populations of fluorescent species within the fluorescence decay maps. Our results demonstrate that this approach allows distinguishing different binders when mixed with the same pigment as well as discriminating different pigments dispersed in a common binder. The results obtained could establish a framework for the analysis of a broader range of pigments and binders to be then extended to several other materials used in art production. The obtained results, together with the compactness and portability of the instrument, pave the way for future in situ applications of the technology on paintings.
Keywords: Gaussian mixture model; TCSPC; binders; cultural heritage; fluorescence lifetime imaging; phasor analysis; pigments; time-resolved fluorescence imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
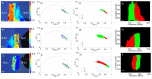



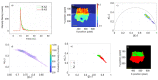
Similar articles
-
In situ noninvasive study of artworks: the MOLAB multitechnique approach.Acc Chem Res. 2010 Jun 15;43(6):728-38. doi: 10.1021/ar100010t. Acc Chem Res. 2010. PMID: 20450184
-
Spectral Behavior of White Pigment Mixtures Using Reflectance, Ultraviolet-Fluorescence Spectroscopy, and Multispectral Imaging.Appl Spectrosc. 2017 Dec;71(12):2616-2625. doi: 10.1177/0003702817717969. Epub 2017 Jul 21. Appl Spectrosc. 2017. PMID: 28730846
-
Palaeoproteomics guidelines to identify proteinaceous binders in artworks following the study of a 15th-century painting by Sandro Botticelli's workshop.Sci Rep. 2022 Jun 23;12(1):10638. doi: 10.1038/s41598-022-14109-w. Sci Rep. 2022. PMID: 35739140 Free PMC article.
-
The Phasor Plot: A Universal Circle to Advance Fluorescence Lifetime Analysis and Interpretation.Annu Rev Biophys. 2021 May 6;50:575-593. doi: 10.1146/annurev-biophys-062920-063631. Annu Rev Biophys. 2021. PMID: 33957055 Review.
-
Linear Combination Properties of the Phasor Space in Fluorescence Imaging.Sensors (Basel). 2022 Jan 27;22(3):999. doi: 10.3390/s22030999. Sensors (Basel). 2022. PMID: 35161742 Free PMC article. Review.
Cited by
-
Visualising varnish removal for conservation of paintings by fluorescence lifetime imaging (FLIM).Herit Sci. 2023;11(1):127. doi: 10.1186/s40494-023-00957-w. Epub 2023 Jun 16. Herit Sci. 2023. PMID: 37333623 Free PMC article.
References
-
- Lakowicz J.R. Principles of Fluorescence Spectroscopy. 3rd ed. Springer; New York, NY, USA: 2008.
-
- Hansell P., Lunnon R.J. Ultraviolet and Fluorescence Recording. Photogr. Sci. Acad. Press; London, UK: 1984. pp. 321–354.
-
- Comelli D., Valentini G., Cubeddu R., Toniolo L. Fluorescence lifetime imaging for the analysis of works of art: Application to fresco paintings and marble sculptures. J. Neutron Res. 2006;14:81–90. doi: 10.1080/10238160600673524. - DOI
-
- de la Rie E.R. Fluorescence of paint and varnish layers (part III) Stud. Conserv. 1982;27:102–108.
-
- Ghirardello M., Valentini G., Toniolo L., Alberti R., Gironda M., Comelli D. Photoluminescence imaging of modern paintings: There is plenty of information at the microsecond timescale. Microchem. J. 2020;154:104618. doi: 10.1016/j.microc.2020.104618. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

