Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes
- PMID: 35268613
- PMCID: PMC8912047
- DOI: 10.3390/molecules27051507
Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes
Abstract
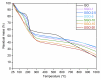
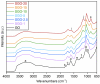
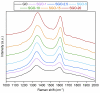
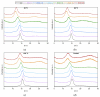
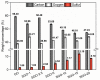
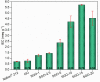
This work deals with the development of graphene oxide (GO)-based self-assembling membranes as possible innovative proton conductors to be used in polymer electrolyte membrane fuel cells (PEMFCs). Nowadays, the most adopted electrolyte is Chemours' Nafion; however, it reveals significant deficiencies such as strong dehydration at high temperature and low humidity, which drastically reduces its proton conductivity. The presence of oxygenated moieties in the GO framework makes it suitable for functionalization, which is required to enhance the promising, but insufficient, proton-carrying features of GO. In this study, sulfonic acid groups (-SO3H) that should favor proton transport were introduced in the membrane structure via a reaction between GO and concentrated sulfuric acid. Six acid-to-GO molar ratios were adopted in the synthesis procedure, giving rise to final products with different sulfonation degrees. All the prepared samples were characterized by means of TGA, ATR-FTIR and Raman spectroscopy, temperature-dependent XRD, SEM and EDX, which pointed out morphological and microstructural changes resulting from the functionalization stage, confirming its effectiveness. Regarding functional features, electrochemical impedance spectroscopy (EIS) as well as measurements of ion exchange capacity (IEC) were carried out to describe the behavior of the various samples, with pristine GO and commercial Nafion® 212 used as reference. EIS tests were performed at five different temperatures (20, 40, 60, 80 and 100 °C) under high (95%) and medium (42%) relative humidity conditions. Compared to both GO and Nafion® 212, the sulfonated specimens demonstrate an increase in the number of ion-carrying groups, as proved by both IEC and EIS tests, which reveal the enhanced proton conductivity of these novel membranes. Specifically, an acid-to-GO molar ratio of 10 produces a six-fold improvement of IEC (4.23 meq g-1) with respect to pure GO (0.76 meq g-1), while a maximum eight-fold improvement (5.72 meq g-1) is achieved in SGO-15.
Keywords: IEC; XRD; graphene oxide; proton conductors; self-assembling membranes; sulfonation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jiang Z., Shi Y., Jiang Z.J., Tian X., Luo L., Chen W. High performance of a free-standing sulfonic acid functionalized holey graphene oxide paper as a proton conducting polymer electrolyte for air-breathing direct methanol fuel cells. J. Mater. Chem. A. 2014;2:6494–6503. doi: 10.1039/c4ta00208c. - DOI
-
- Claramunt S., Varea A., López-Díaz D., Velázquez M.M., Cornet A., Cirera A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C. 2015;119:10123–10129. doi: 10.1021/acs.jpcc.5b01590. - DOI
-
- Tian Y., Yu Z., Cao L., Zhang X.L., Sun C., Wang D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021;55:323–344. doi: 10.1016/j.jechem.2020.07.006. - DOI
-
- Smith A.T., LaChance A.M., Zeng S., Liu B., Sun L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019;1:31–47. doi: 10.1016/j.nanoms.2019.02.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

