A Multi-Technique Investigation of the Complex Formation Equilibria between Bis-Deferiprone Derivatives and Oxidovanadium (IV)
- PMID: 35268654
- PMCID: PMC8924880
- DOI: 10.3390/molecules27051555
A Multi-Technique Investigation of the Complex Formation Equilibria between Bis-Deferiprone Derivatives and Oxidovanadium (IV)
Abstract
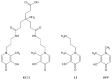
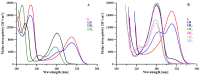
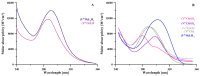
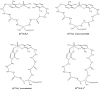
The increasing biomedical interest in high-stability oxidovanadium(IV) complexes with hydroxypyridinone ligands leads us to investigate the complex formation equilibria of VIVO2+ ion with a tetradentate ligand, named KC21, which contains two 3-hydroxy-1,2-dimethylpyridin-4(1H)-one (deferiprone) moieties, and with the simple bidentate ligand that constitutes the basic unit of KC21, for comparison, named L5. These equilibrium studies were conducted with joined potentiometric-spectrophotometric titrations, and the results were substantiated with EPR measurements at variable pH values. This multi-technique study gave evidence of the formation of an extremely stable 1:1 complex between KC21 and oxidovanadium(IV) at a physiological pH, which could find promising pharmacological applications.
Keywords: EPR spectroscopy; UV spectrophotometry; deferiprone; oxidovanadium (IV); potentiometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Structural study of the interaction of vanadate with the ligand 1,2-dimethyl-3-hydroxy-4-pyridinone (Hdmpp) in aqueous solution.J Inorg Biochem. 2000 May 30;80(1-2):177-9. doi: 10.1016/s0162-0134(00)00028-3. J Inorg Biochem. 2000. PMID: 10885483
-
Spectroscopic and potentiometric characterization of oxovanadium(IV) complexes formed by 3-hydroxy-4-pyridinones. Rationalization of the influence of basicity and electronic structure of the ligand on the properties of V(IV)O species in aqueous solution.Inorg Chem. 2006 Oct 2;45(20):8086-97. doi: 10.1021/ic0605571. Inorg Chem. 2006. PMID: 16999406
-
Thermodynamic Study of Oxidovanadium(IV) with Kojic Acid Derivatives: A Multi-Technique Approach.Pharmaceuticals (Basel). 2021 Oct 12;14(10):1037. doi: 10.3390/ph14101037. Pharmaceuticals (Basel). 2021. PMID: 34681261 Free PMC article.
-
Assemblies of salen-type oxidovanadium(IV) complexes: substituent effects and in vitro protein tyrosine phosphatase inhibition.Dalton Trans. 2014 Dec 7;43(45):17044-53. doi: 10.1039/c4dt02344g. Dalton Trans. 2014. PMID: 25303031
-
On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World.Molecules. 2020 Jul 6;25(13):3073. doi: 10.3390/molecules25133073. Molecules. 2020. PMID: 32640541 Free PMC article. Review.
References
-
- Crans D.C., Yang L., Haase A., Yang X. Health Benefits of Vanadium and Its Potential as an Anticancer Agent. In: Sigel A., Sigel H., Freisinger E., Sigel R.K.O., editors. Metallo-Drugs: Development and Action of Anticancer Agents. De Gruyter GmbH; Berlin, Germany: 2018. pp. 251–280.
-
- Treviño S., Díaz A., Sánchez-Lara E., Sanchez-Gaytan B.L., Perez-Aguilar J.M., González-Vergara E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Element Res. 2019;188:68–98. doi: 10.1007/s12011-018-1540-6. - DOI - PMC - PubMed
-
- Crans D.C., Henry L., Cardiff G., Posner B.I. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective. In: Carver P.L., editor. Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic. 1st ed. De Gruyter; Berlin, Germany: Boston, MA, USA: 2019. pp. 203–230. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

