Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study
- PMID: 35268796
- PMCID: PMC8911599
- DOI: 10.3390/molecules27051694
Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study
Abstract
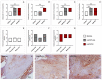
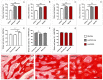
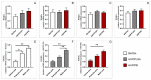
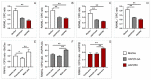
Reconstruction of jaw bone defects present a significant problem because of specific aesthetic and functional requirements. Although widely used, the transplantation of standard autograft and allograft materials is still associated with significant constraints. Composite scaffolds, combining advantages of biodegradable polymers with bioceramics, have potential to overcome limitations of standard grafts. Polyethyleneimine could be an interesting novel biocompatible polymer for scaffold construction due to its biocompatibility and chemical structure. To date, there have been no in vivo studies assessing biological properties of hydroxyapatite bioceramics scaffold modified with polyethyleneimine. The aim of this study was to evaluate in vivo effects of composite scaffolds of hydroxyapatite ceramics and poly(lactide-co-glycolide) and novel polyethyleneimine on bone repair in swine's mandibular defects, and to compare them to conventional bone allograft (BioOss). Scaffolds were prepared using the method of polymer foam template in three steps. Pigs, 3 months old, were used and defects were made in the canine, premolar, and molar area of their mandibles. Four months following the surgical procedure, the bone was analyzed using radiological, histological, and gene expression techniques. Hydroxyapatite ceramics/polyethyleneimine composite scaffold demonstrated improved biological behavior compared to conventional allograft in treatment of swine's mandibular defects, in terms of bone density and bone tissue histological characteristics.
Keywords: composite scaffolds; hydroxyapatite ceramics; mandibular defect; poly(lactide-co-glycolide); polyethyleneimine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Probst F.A., Fliefel R., Burian E., Probst M., Eddicks M., Cornelsen M., Riedl C., Seitz H., Aszódi A., Schieker M., et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-59038-8. - DOI - PMC - PubMed
-
- Jokanović V., Čolović B., Marković D., Petrović M., Soldatović I., Antonijević D., Milosavljević P., Sjerobabin N., Sopta J. Extraordinary biological properties of a new calcium hydroxyapatite/poly(lactide-co-glycolide)-based scaffold confirmed by in vivo investigation. Biomed. Eng. Biomed. Tech. 2017;62:295–306. doi: 10.1515/bmt-2015-0164. - DOI - PubMed
-
- Micic M., Antonijevic D., Milutinovic-Smiljanic S., Trisic D., Colovic B., Kosanovic D., Prokic B., Vasic J., Zivkovic S., Milasin J., et al. Developing a novel resorptive hydroxyapatite-based bone substitute for over-critical size defect reconstruction: Physicochemical and biological characterization and proof of concept in segmental rabbit’s ulna reconstruction. Biomed. Eng. Biomed. Tech. 2020;65:491–505. doi: 10.1515/bmt-2019-0218. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

