Inhibition of the Quorum Sensing System, Elastase Production and Biofilm Formation in Pseudomonas aeruginosa by Psammaplin A and Bisaprasin
- PMID: 35268822
- PMCID: PMC8911947
- DOI: 10.3390/molecules27051721
Inhibition of the Quorum Sensing System, Elastase Production and Biofilm Formation in Pseudomonas aeruginosa by Psammaplin A and Bisaprasin
Abstract
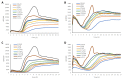
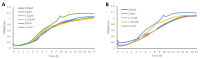
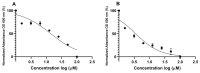
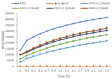
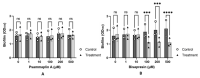
Natural products derived from marine sponges have exhibited bioactivity and, in some cases, serve as potent quorum sensing inhibitory agents that prevent biofilm formation and attenuate virulence factor expression by pathogenic microorganisms. In this study, the inhibitory activity of the psammaplin-type compounds, psammaplin A (1) and bisaprasin (2), isolated from the marine sponge, Aplysinellarhax, are evaluated in quorum sensing inhibitory assays based on the Pseudomonas aeruginosa PAO1 lasB-gfp(ASV) and rhlA-gfp(ASV) biosensor strains. The results indicate that psammaplin A (1) showed moderate inhibition on lasB-gfp expression, but significantly inhibited the QS-gene promoter, rhlA-gfp, with IC50 values at 14.02 μM and 4.99 μM, respectively. In contrast, bisaprasin (2) displayed significant florescence inhibition in both biosensors, PAO1 lasB-gfp and rhlA-gfp, with IC50 values at 3.53 μM and 2.41 μM, respectively. Preliminary analysis suggested the importance of the bromotyrosine and oxime functionalities for QSI activity in these molecules. In addition, psammaplin A and bisaprasin downregulated elastase expression as determined by the standard enzymatic elastase assay, although greater reduction in elastase production was observed with 1 at 50 μM and 100 μM. Furthermore, the study revealed that bisaprasin (2) reduced biofilm formation in P. aeruginosa.
Keywords: Pseudomonas aeruginosa; elastase inhibitor; inhibitor of biofilm formation; marine natural products; marine sponge; psammaplin; quorum sensing inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Markwart R., Willrich N., Haller S., Noll I., Koppe U., Werner G., Eckmanns T., Reuss A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German antimicrobial resistance surveillance (ARS) Antimicrob. Resist. Infect. Control. 2019;8:147. doi: 10.1186/s13756-019-0594-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

