Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis
- PMID: 35268859
- PMCID: PMC8911232
- DOI: 10.3390/ma15051628
Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis
Abstract
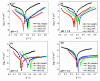
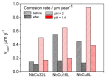
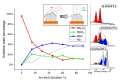
For proton exchange membrane water electrolysis (PEMWE) to become competitive, the cost of stack components, such as bipolar plates (BPP), needs to be reduced. This can be achieved by using coated low-cost materials, such as copper as alternative to titanium. Herein we report on highly corrosion-resistant copper BPP coated with niobium. All investigated samples showed excellent corrosion resistance properties, with corrosion currents lower than 0.1 µA cm-2 in a simulated PEM electrolyzer environment at two different pH values. The physico-chemical properties of the Nb coatings are thoroughly characterized by scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS), X-ray photoelectron spectroscopy (XPS), and atomic force microscopy (AFM). A 30 µm thick Nb coating fully protects the Cu against corrosion due to the formation of a passive oxide layer on its surface, predominantly composed of Nb2O5. The thickness of the passive oxide layer determined by both EIS and XPS is in the range of 10 nm. The results reported here demonstrate the effectiveness of Nb for protecting Cu against corrosion, opening the possibility to use it for the manufacturing of BPP for PEMWE. The latter was confirmed by its successful implementation in a single cell PEMWE based on hydraulic compression technology.
Keywords: PEMWE; bipolar plate; coatings; corrosion resistance; cost reduction; water electrolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Press R.J., Santhanam K.S.V., Miri M.J., Bailey A.V., Takacs G.A. Introduction to Hydrogen Technology. John Wiley & Sons; Hoboken, NJ, USA: 2009. p. 211.
-
- Dincer I., Acar C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy. 2015;40:11094–11111. doi: 10.1016/j.ijhydene.2014.12.035. - DOI
-
- Wittstadt U. Electrolysis: Hydrogen production using electricity. In: Züttel A., Borgschulte A., Schlapbach L., editors. Hydrogen as a Future Energy Carrier. Wiley-VCH; Weinheim, Germany: 2008. p. 155.
-
- Millet P., Ngameni R., Grigoriev S.A., Mbemba N., Brisset F., Ranjbari A., Etiévant C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrogen Energy. 2010;35:5043–5052. doi: 10.1016/j.ijhydene.2009.09.015. - DOI
-
- Langemann M., Fritz D.L., Müller M., Stolten D. Validation and characterization of suitable materials for bipolar plates in PEM water electrolysis. Int. J. Hydrogen Energy. 2015;40:11385–11391. doi: 10.1016/j.ijhydene.2015.04.155. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

