Modified Histopathological Protocol for Poly-ɛ-Caprolactone Scaffolds Preserving Their Trabecular, Honeycomb-like Structure
- PMID: 35268968
- PMCID: PMC8911251
- DOI: 10.3390/ma15051732
Modified Histopathological Protocol for Poly-ɛ-Caprolactone Scaffolds Preserving Their Trabecular, Honeycomb-like Structure
Abstract
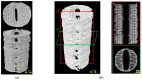
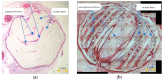
Poly-ɛ-caprolactone (PCL) is now widely studied in relation to the engineering of bone, cartilage, tendons, and other tissues. Standard histological protocols can destroy the carefully created trabecular and honeycomb-like architecture of PCL scaffolds, and could lead to scaffold fibers swelling, resulting in the displacement or compression of tissues inside the scaffold. The aim of this study was to modify a standard histopathological protocol for PCL scaffold preparation and evaluate it on porous cylindrical PCL scaffolds in a rat model. In 16 inbred Wag rats, 2 PCL scaffolds were implanted subcutaneously to both inguinal areas. Two months after implantation, harvested scaffolds were first subjected to μCT imaging, and then to histopathological analysis with standard (left inguinal area) and modified histopathological protocols (right inguinal area). To standardize the results, soft tissue percentages (STPs) were calculated on scaffold cross-sections obtained from both histopathological protocols and compared with corresponding µCT cross-sections. The modified protocol enabled the assessment of almost 10× more soft tissues on the scaffold cross-section than the standard procedure. Moreover, STP was only 1.5% lower than in the corresponding µCT cross-sections assessed before the histopathological procedure. The presented modification of the histopathological protocol is cheap, reproducible, and allows for a comprehensive evaluation of PCL scaffolds while maintaining their trabecular, honeycomb-like structure on cross-sections.
Keywords: PCL; full cross-section of scaffold; histopathological protocol; scaffold; scaffold architecture preservation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures











References
-
- van Natta F.J., Hill J.W., Carothers W.H. Studies of Polymerization and Ring Formation. XXIII.1 ε-Caprolactone and its Polymers. J. Am. Chem. Soc. 1934;56:455–457. doi: 10.1021/ja01317a053. - DOI
-
- Chlanda A., Kijeńska-Gawrońska E., Zdunek J., Swieszkowski W. Internal nanocrystalline structure and stiffness alterations of electrospun polycaprolactone-based mats after six months of in vitro degradation. An atomic force microscopy assay. J. Mech. Behav. Biomed. Mater. 2020;101:103437. doi: 10.1016/j.jmbbm.2019.103437. - DOI - PubMed
-
- Augustine R., Nethi S.K., Kalarikkal N., Thomas S., Patra C.R. Electrospun polycaprolactone (PCL) scaffolds embedded with europium hydroxide nanorods (EHNs) with enhanced vascularization and cell proliferation for tissue engineering applications. J. Mater. Chem. B. 2017;5:4660–4672. doi: 10.1039/C7TB00518K. - DOI - PubMed
-
- Sajkiewicz P., Heljak M., Gradys A., Choińska E., Rumiński S., Jaroszewicz T., Bissenik I., Swieszkowski W. Degradation and related changes in supermolecular structure of poly(caprolactone) in vivo conditions. Polym. Degrad. Stab. 2018;157:70–79. doi: 10.1016/j.polymdegradstab.2018.09.023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

