Three-Dimensional Printing of a Hybrid Bioceramic and Biopolymer Porous Scaffold for Promoting Bone Regeneration Potential
- PMID: 35269209
- PMCID: PMC8911960
- DOI: 10.3390/ma15051971
Three-Dimensional Printing of a Hybrid Bioceramic and Biopolymer Porous Scaffold for Promoting Bone Regeneration Potential
Abstract
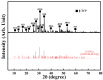
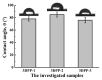
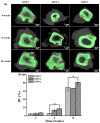
In this study, we proposed a three-dimensional (3D) printed porous (termed as 3DPP) scaffold composed of bioceramic (beta-tricalcium phosphate (β-TCP)) and thermoreversible biopolymer (pluronic F-127 (PF127)) that may provide bone tissue ingrowth and loading support for bone defect treatment. The investigated scaffolds were printed in three different ranges of pore sizes for comparison (3DPP-1: 150−200 μm, 3DPP-2: 250−300 μm, and 3DPP-3: 300−350 μm). The material properties and biocompatibility of the 3DPP scaffolds were characterized using scanning electron microscopy, X-ray diffractometry, contact angle goniometry, compression testing, and cell viability assay. In addition, micro-computed tomography was applied to investigate bone regeneration behavior of the 3DPP scaffolds in the mini-pig model. Analytical results showed that the 3DPP scaffolds exhibited well-defined porosity, excellent microstructural interconnectivity, and acceptable wettability (θ < 90°). Among all groups, the 3DPP-1 possessed a significantly highest compressive force 273 ± 20.8 Kgf (* p < 0.05). In vitro experiment results also revealed good cell viability and cell attachment behavior in all 3DPP scaffolds. Furthermore, the 3DPP-3 scaffold showed a significantly higher percentage of bone formation volume than the 3DPP-1 scaffold at week 8 (* p < 0.05) and week 12 (* p < 0.05). Hence, the 3DPP scaffold composed of β-TCP and F-127 is a promising candidate to promote bone tissue ingrowth into the porous scaffold with decent biocompatibility. This scaffold particularly fabricated with a pore size of around 350 μm (i.e., 3DPP-3 scaffold) can provide proper loading support and promote bone regeneration in bone defects when applied in dental and orthopedic fields.
Keywords: 3D printing; biocompatibility; bone regeneration; pluronic F127; tricalcium phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds.Acta Biomater. 2016 Feb;31:401-411. doi: 10.1016/j.actbio.2015.11.050. Epub 2015 Nov 30. Acta Biomater. 2016. PMID: 26621692
-
3D-printed MgO nanoparticle loaded polycaprolactone β-tricalcium phosphate composite scaffold for bone tissue engineering applications: In-vitro and in-vivo evaluation.J Biomed Mater Res A. 2023 Mar;111(3):322-339. doi: 10.1002/jbm.a.37465. Epub 2022 Nov 5. J Biomed Mater Res A. 2023. PMID: 36334300
-
3D printed bioceramic scaffolds: Adjusting pore dimension is beneficial for mandibular bone defects repair.J Tissue Eng Regen Med. 2022 Apr;16(4):409-421. doi: 10.1002/term.3287. Epub 2022 Feb 14. J Tissue Eng Regen Med. 2022. PMID: 35156316
-
[Biological evaluation of three-dimensional printed co-poly lactic acid/glycolic acid/tri-calcium phosphate scaffold for bone reconstruction].Zhonghua Kou Qiang Yi Xue Za Zhi. 2016 Nov 9;51(11):661-666. doi: 10.3760/cma.j.issn.1002-0098.2016.11.005. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016. PMID: 27806758 Chinese.
-
3D powder printed tetracalcium phosphate scaffold with phytic acid binder: fabrication, microstructure and in situ X-Ray tomography analysis of compressive failure.J Mater Sci Mater Med. 2018 Mar 8;29(3):29. doi: 10.1007/s10856-018-6034-8. J Mater Sci Mater Med. 2018. PMID: 29520670
Cited by
-
Biomimetic structural design in 3D-printed scaffolds for bone tissue engineering.Mater Today Bio. 2025 Mar 14;32:101664. doi: 10.1016/j.mtbio.2025.101664. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40206144 Free PMC article. Review.
-
Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions.J Funct Biomater. 2024 Sep 25;15(10):280. doi: 10.3390/jfb15100280. J Funct Biomater. 2024. PMID: 39452579 Free PMC article. Review.
-
PF127 Hydrogel-Based Delivery of Exosomal CTNNB1 from Mesenchymal Stem Cells Induces Osteogenic Differentiation during the Repair of Alveolar Bone Defects.Nanomaterials (Basel). 2023 Mar 16;13(6):1083. doi: 10.3390/nano13061083. Nanomaterials (Basel). 2023. PMID: 36985977 Free PMC article.
-
Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones.Biomedicines. 2023 Jan 24;11(2):325. doi: 10.3390/biomedicines11020325. Biomedicines. 2023. PMID: 36830862 Free PMC article. Review.
-
Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment.Materials (Basel). 2022 May 5;15(9):3319. doi: 10.3390/ma15093319. Materials (Basel). 2022. PMID: 35591656 Free PMC article.
References
-
- Sun H., Hu C., Zhou C., Wu L., Sun J., Zhou X., Xing F., Long C., Kong Q., Liang J., et al. 3D printing of calcium phosphate scaffolds with controlled release of antibacterial functions for jaw bone repair. Mater. Des. 2020;189:108540. doi: 10.1016/j.matdes.2020.108540. - DOI
-
- Jacobson J.A., Yanoso-Scholl L., Reynolds D.G., Dadali T., Bradica G., Bukata S., Puzas E.J., Zuscik M.J., Rosier R., O’Keefe R.J., et al. Teriparatide therapy and beta-tricalcium phosphate enhance scaffold reconstruction of mouse femoral defects. Tissue Eng. Part A. 2011;17:389–398. doi: 10.1089/ten.tea.2010.0115. - DOI - PMC - PubMed
-
- Horowitz R.A., Mazor Z., Foitzik C., Prasad H., Rohrer M., Palti A. β-tricalcium phosphate as bone substitute material: Properties and clinical applications. J. Osseointegr. 2010;2:61–68.
-
- Gao P., Zhang H., Liu Y., Fan B., Li X., Xiao X., Lan P., Li M., Geng L., Liu D., et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep. 2016;6:23367. doi: 10.1038/srep23367. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

