Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays
- PMID: 35269259
- PMCID: PMC8911739
- DOI: 10.3390/nano12050771
Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays
Abstract
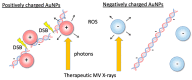
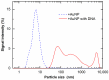
Gold nanoparticles (AuNPs) can be used with megavolt (MV) X-rays to exert radiosensitization effects, as demonstrated in cell survival assays and mouse experiments. However, the detailed mechanisms are not clear; besides physical dose enhancement, several chemical and biological processes have been proposed. Reducing the AuNP concentration while achieving sufficient enhancement is necessary for the clinical application of AuNPs. Here, we used positively charged (+) AuNPs to determine the radiosensitization effects of AuNPs combined with MV X-rays on DNA damage in vitro. We examined the effect of low concentrations of AuNPs on DNA damage and reactive oxygen species (ROS) generation. DNA damage was promoted by 1.4 nm +AuNP with dose enhancement factors of 1.4 ± 0.2 for single-strand breaks and 1.2 ± 0.1 for double-strand breaks. +AuNPs combined with MV X-rays induced radiosensitization at the DNA level, indicating that the effects were physical and/or chemical. Although -AuNPs induced similar ROS levels, they did not cause considerable DNA damage. Thus, dose enhancement by low concentrations of +AuNPs may have occurred with the increase in the local +AuNP concentration around DNA or via DNA binding. +AuNPs showed stronger radiosensitization effects than -AuNPs. Combining +AuNPs with MV X-rays in radiation therapy may improve clinical outcomes.
Keywords: DNA damage; MV X-rays; gold nanoparticle; positively charged nanoparticle; radiation therapy; radiosensitizer.
Conflict of interest statement
The authors declare no conflict of interest in this work.
Figures






Similar articles
-
An Evaluation of the Potential Radiosensitization Effect of Spherical Gold Nanoparticles to Induce Cellular Damage Using Different Radiation Qualities.Molecules. 2025 Feb 24;30(5):1038. doi: 10.3390/molecules30051038. Molecules. 2025. PMID: 40076263 Free PMC article.
-
Effect of Gold Nanoparticle Radiosensitization on Plasmid DNA Damage Induced by High-Dose-Rate Brachytherapy.Int J Nanomedicine. 2021 Jan 14;16:359-370. doi: 10.2147/IJN.S292105. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33469290 Free PMC article.
-
Quantifying Radiosensitization of PSMA-Targeted Gold Nanoparticles on Prostate Cancer Cells at Megavoltage Radiation Energies by Monte Carlo Simulation and Local Effect Model.Pharmaceutics. 2022 Oct 17;14(10):2205. doi: 10.3390/pharmaceutics14102205. Pharmaceutics. 2022. PMID: 36297640 Free PMC article.
-
Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements.Adv Drug Deliv Rev. 2017 Jan 15;109:84-101. doi: 10.1016/j.addr.2015.12.012. Epub 2015 Dec 19. Adv Drug Deliv Rev. 2017. PMID: 26712711 Review.
-
Radiosensitization by gold nanoparticles: Will they ever make it to the clinic?Radiother Oncol. 2017 Sep;124(3):344-356. doi: 10.1016/j.radonc.2017.07.007. Epub 2017 Aug 4. Radiother Oncol. 2017. PMID: 28784439 Review.
Cited by
-
An Evaluation of the Potential Radiosensitization Effect of Spherical Gold Nanoparticles to Induce Cellular Damage Using Different Radiation Qualities.Molecules. 2025 Feb 24;30(5):1038. doi: 10.3390/molecules30051038. Molecules. 2025. PMID: 40076263 Free PMC article.
-
Monte Carlo simulation of physical dose enhancement in core-shell magnetic gold nanoparticles with TOPAS.Front Oncol. 2022 Sep 14;12:992358. doi: 10.3389/fonc.2022.992358. eCollection 2022. Front Oncol. 2022. PMID: 36185221 Free PMC article.
-
Gold Nanoparticle-Enhanced Production of Reactive Oxygen Species for Radiotherapy and Phototherapy.Nanomaterials (Basel). 2025 Feb 19;15(4):317. doi: 10.3390/nano15040317. Nanomaterials (Basel). 2025. PMID: 39997879 Free PMC article. Review.
-
Shape-Driven Response of Gold Nanoparticles to X-rays.Nanomaterials (Basel). 2023 Oct 7;13(19):2719. doi: 10.3390/nano13192719. Nanomaterials (Basel). 2023. PMID: 37836360 Free PMC article.
-
Mechanisms of Nanoscale Radiation Enhancement by Metal Nanoparticles: Role of Low Energy Electrons.Int J Mol Sci. 2023 Feb 28;24(5):4697. doi: 10.3390/ijms24054697. Int J Mol Sci. 2023. PMID: 36902132 Free PMC article. Review.
References
-
- Khan F.M., Gibbons J.P. Khan’s The Physics of Radiation Therapy. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2014. pp. 430–453.
-
- Ezzell G.A., Galvin J.M., Low D., Palta J.R., Rosen I., Sharpe M.B., Xia P., Xiao Y., Xing L., Yu C.X. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med. Phys. 2003;30:2089–2115. doi: 10.1118/1.1591194. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

