Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix
- PMID: 35269382
- PMCID: PMC8909158
- DOI: 10.3390/cells11050760
Cerebral Organoids Maintain the Expression of Neural Stem Cell-Associated Glycoepitopes and Extracellular Matrix
Abstract
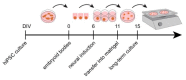
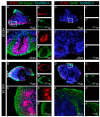
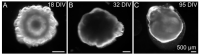
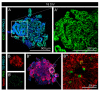
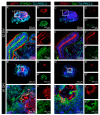
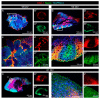
During development, the nervous system with its highly specialized cell types forms from a pool of relatively uniform stem cells. This orchestrated process requires tight regulation. The extracellular matrix (ECM) is a complex network rich in signaling molecules, and therefore, of interest in this context. Distinct carbohydrate structures, bound to ECM molecules like Tenascin C (TNC), are associated with neural stem/progenitor cells. We have analyzed the expression patterns of the LewisX (LeX) trisaccharide motif and of the sulfation-dependent DSD-1 chondroitin sulfate glycosaminoglycan epitope in human cerebral organoids, a 3D model for early central nervous system (CNS) development, immunohistochemically. In early organoids we observed distinct expression patterns of the glycoepitopes, associated with rosette-like structures that resemble the neural tube in vitro: Terminal LeX motifs, recognized by the monoclonal antibody (mAb) 487LeX, were enriched in the lumen and at the outer border of neural rosettes. In contrast, internal LeX motif repeats detected with mAb 5750LeX were concentrated near the lumen. The DSD-1 epitope, labeled with mAb 473HD, was detectable at rosette borders and in adjacent cells. The epitope expression was maintained in older organoids but appeared more diffuse. The differential glycoepitope expression suggests a specific function in the developing human CNS.
Keywords: DSD-1 epitope; LewisX epitope; PTPRZ1; Tenascin C; cerebral organoid; chondroitin sulfate; extracellular matrix; glycoepitope; neural stem cell; radial glia.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Glycoconjugates reveal diversity of human neural stem cells (hNSCs) derived from human induced pluripotent stem cells (hiPSCs).Cell Tissue Res. 2017 Jun;368(3):531-549. doi: 10.1007/s00441-017-2594-z. Epub 2017 Mar 15. Cell Tissue Res. 2017. PMID: 28299522
-
The DSD-1 carbohydrate epitope depends on sulfation, correlates with chondroitin sulfate D motifs, and is sufficient to promote neurite outgrowth.J Biol Chem. 1998 Oct 23;273(43):28444-53. doi: 10.1074/jbc.273.43.28444. J Biol Chem. 1998. PMID: 9774473
-
Tenascin-C in the matrisome of neural stem and progenitor cells.Mol Cell Neurosci. 2017 Jun;81:22-31. doi: 10.1016/j.mcn.2016.11.003. Epub 2016 Nov 9. Mol Cell Neurosci. 2017. PMID: 27836730 Review.
-
The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation.J Neurosci. 2006 Apr 12;26(15):4082-94. doi: 10.1523/JNEUROSCI.0422-06.2006. J Neurosci. 2006. PMID: 16611825 Free PMC article.
-
The Use of Pluripotent Stem Cell-Derived Organoids to Study Extracellular Matrix Development during Neural Degeneration.Cells. 2019 Mar 14;8(3):242. doi: 10.3390/cells8030242. Cells. 2019. PMID: 30875781 Free PMC article. Review.
Cited by
-
Overview of Neural Tube Defects: Gene-Environment Interactions, Preventative Approaches and Future Perspectives.Biomedicines. 2022 Apr 21;10(5):965. doi: 10.3390/biomedicines10050965. Biomedicines. 2022. PMID: 35625701 Free PMC article. Review.
-
Frontiers in Neurogenesis.Cells. 2022 Nov 11;11(22):3567. doi: 10.3390/cells11223567. Cells. 2022. PMID: 36428996 Free PMC article.
-
Hindbrain boundaries as niches of neural progenitor and stem cells regulated by the extracellular matrix proteoglycan chondroitin sulphate.Development. 2024 Feb 15;151(4):dev201934. doi: 10.1242/dev.201934. Epub 2024 Feb 13. Development. 2024. PMID: 38251863 Free PMC article.
-
Spatio-temporal dynamics enhance cellular diversity, neuronal function and further maturation of human cerebral organoids.Commun Biol. 2023 Feb 14;6(1):173. doi: 10.1038/s42003-023-04547-1. Commun Biol. 2023. PMID: 36788328 Free PMC article.
-
HCMV Infection Reduces Nidogen-1 Expression, Contributing to Impaired Neural Rosette Development in Brain Organoids.J Virol. 2023 May 31;97(5):e0171822. doi: 10.1128/jvi.01718-22. Epub 2023 May 1. J Virol. 2023. PMID: 37125912 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

