C1q/TNF-Related Protein 3 Prevents Diabetic Retinopathy via AMPK-Dependent Stabilization of Blood-Retinal Barrier Tight Junctions
- PMID: 35269401
- PMCID: PMC8909652
- DOI: 10.3390/cells11050779
C1q/TNF-Related Protein 3 Prevents Diabetic Retinopathy via AMPK-Dependent Stabilization of Blood-Retinal Barrier Tight Junctions
Abstract
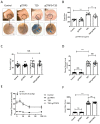
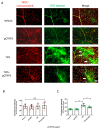
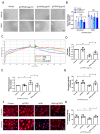
Background The impairment of the inner blood-retinal barrier (iBRB) increases the pathological development of diabetic retinopathy (DR), a severe complication in diabetic patients. Identifying approaches to preserving iBRB integrity and function is a significant challenge in DR. C1q/tumor necrosis factor-related protein-3 (CTRP3) is a newly discovered adipokine and a vital biomarker, predicting DR severity. We sought to determine whether and how CTRP3 affects the pathological development of non-proliferative diabetic retinopathy (NPDR). Methods To clarify the pathophysiologic progress of the blood-retinal barrier in NPDR and explore its potential mechanism, a mouse Type 2 diabetic model of diabetic retinopathy was used. The capillary leakage was assessed by confocal microscope with fluorescent-labeled protein in vivo. Furthermore, the effect of CTRP3 on the inner blood-retinal barrier (iBRB) and its molecular mechanism was clarified. Results The results demonstrated that CTRP3 protects iBRB integrity and resists the vascular permeability induced by DR. Mechanistically, the administration of CTRP3 activates the AMPK signaling pathway and enhances the expression of Occludin and Claudin-5 (tight junction protein) in vivo and in vitro. Meanwhile, CTRP3 improves the injury of human retinal endothelial cells (HRMECs) induced by high glucose/high lipids (HG/HL), and its protective effects are AMPK-dependent. Conclusions In summary, we report, for the first time, that CTRP3 prevents diabetes-induced retinal vascular permeability via stabilizing the tight junctions of the iBRB and through the AMPK-dependent Occludin/Claudin-5 signaling pathway, thus critically affecting the development of NPDR.
Keywords: CTRP3; diabetic retinopathy; iBRB; permeability; tight junction proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Qaum T., Xu Q., Joussen A.M., Clemens M.W., Qin W., Miyamoto K., Hassessian H., Wiegand S.J., Rudge J., Yancopoulos G.D. VEGF-initiated blood–retinal barrier breakdown in early diabetes. Investig. Ophthalmol. Vis. Sci. 2001;42:2408–2413. - PubMed
-
- El-Remessy A.B., Behzadian M.A., Abou-Mohamed G., Franklin T., Caldwell R.W., Caldwell R.B. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am. J. Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

