Experimental Arthritis Inhibits Adult Hippocampal Neurogenesis in Mice
- PMID: 35269413
- PMCID: PMC8909078
- DOI: 10.3390/cells11050791
Experimental Arthritis Inhibits Adult Hippocampal Neurogenesis in Mice
Abstract
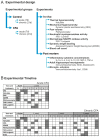
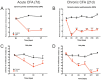
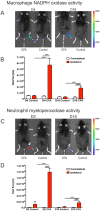
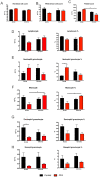
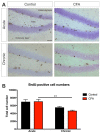
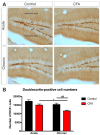
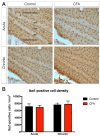
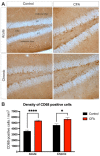
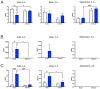
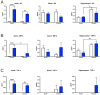
Background: Adult-born neurons of the hippocampal dentate gyrus play a role in specific forms of learning, and disturbed neurogenesis seems to contribute to the development of neuropsychiatric disorders, such as major depression. Neuroinflammation inhibits adult neurogenesis, but the effect of peripheral inflammation on this form of neuroplasticity is ambiguous. Objective: Our aim was to investigate the influence of acute and chronic experimental arthritis on adult hippocampal neurogenesis and to elucidate putative regulatory mechanisms. Methods: Arthritis was triggered by subcutaneous injection of complete Freund's adjuvant (CFA) into the hind paws of adult male mice. The animals were killed either seven days (acute inflammation) or 21 days (chronic inflammation) after the CFA injection. Behavioral tests were used to demonstrate arthritis-related hypersensitivity to painful stimuli. We used in vivo bioluminescence imaging to verify local inflammation. The systemic inflammatory response was assessed by complete blood cell counts and by measurement of the cytokine/chemokine concentrations of TNF-α, IL-1α, IL-4, IL-6, IL-10, KC and MIP-2 in the inflamed hind limbs, peripheral blood and hippocampus to characterize the inflammatory responses in the periphery and in the brain. In the hippocampal dentate gyrus, the total number of newborn neurons was determined with quantitative immunohistochemistry visualizing BrdU- and doublecortin-positive cells. Microglial activation in the dentate gyrus was determined by quantifying the density of Iba1- and CD68-positive cells. Results: Both acute and chronic arthritis resulted in paw edema, mechanical and thermal hyperalgesia. We found phagocytic infiltration and increased levels of TNF-α, IL-4, IL-6, KC and MIP-2 in the inflamed hind paws. Circulating neutrophil granulocytes and IL-6 levels increased in the blood solely during the acute phase. In the dentate gyrus, chronic arthritis reduced the number of doublecortin-positive cells, and we found increased density of CD68-positive macrophages/microglia in both the acute and chronic phases. Cytokine levels, however, were not altered in the hippocampus. Conclusions: Our data suggest that acute peripheral inflammation initiates a cascade of molecular and cellular changes that eventually leads to reduced adult hippocampal neurogenesis, which was detectable only in the chronic inflammatory phase.
Keywords: animal model; cell proliferation; chemokine; chronic inflammation; chronic pain; cytokine; dentate gyrus; hippocampus; microglia; neuroplasticity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; nor in the writing of the manuscript or the decision to publish the results.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

