Activation of Subcutaneous Mast Cells in Acupuncture Points Triggers Analgesia
- PMID: 35269431
- PMCID: PMC8909735
- DOI: 10.3390/cells11050809
Activation of Subcutaneous Mast Cells in Acupuncture Points Triggers Analgesia
Abstract
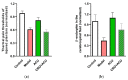
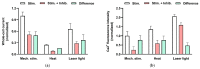
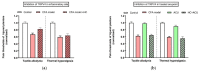
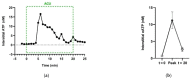
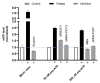
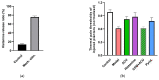
This review summarizes experimental evidence indicating that subcutaneous mast cells are involved in the trigger mechanism of analgesia induced by acupuncture, a traditional oriental therapy, which has gradually become accepted worldwide. The results are essentially based on work from our laboratories. Skin mast cells are present at a high density in acupuncture points where fine needles are inserted and manipulated during acupuncture intervention. Mast cells are sensitive to mechanical stimulation because they express multiple types of mechanosensitive channels, including TRPV1, TRPV2, TRPV4, receptors and chloride channels. Acupuncture manipulation generates force and torque that indirectly activate the mast cells via the collagen network. Subsequently, various mediators, for example, histamine, serotonin, adenosine triphosphate and adenosine, are released from activated mast cells to the interstitial space; they or their downstream products activate the corresponding receptors situated at local nerve terminals of sensory neurons in peripheral ganglia. The analgesic effects are thought to be generated via the reduced electrical activities of the primary sensory neurons. Alternatively, these neurons project such signals to pain-relevant regions in spinal cord and/or higher centers of the brain.
Keywords: TRPV; acupuncture; analgesia; histamine; mast cells; mechanosensitivity; purinergic signals; serotonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Wang X., Yao W., Huang M., Zhang D., Xia Y. Signal Transduction in Acupoints. In: Xia Y., editor. Advanced Acupuncture Research: From Bench to Bedside. Springer; Berlin/Heidelberg, Germany: 2022.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

