Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements
- PMID: 35269432
- PMCID: PMC8909309
- DOI: 10.3390/cells11050810
Transience of the Retinal Output Is Determined by a Great Variety of Circuit Elements
Abstract
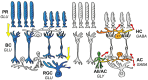
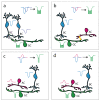
Retinal ganglion cells (RGCs) encrypt stimulus features of the visual scene in action potentials and convey them toward higher visual centers in the brain. Although there are many visual features to encode, our recent understanding is that the ~46 different functional subtypes of RGCs in the retina share this task. In this scheme, each RGC subtype establishes a separate, parallel signaling route for a specific visual feature (e.g., contrast, the direction of motion, luminosity), through which information is conveyed. The efficiency of encoding depends on several factors, including signal strength, adaptational levels, and the actual efficacy of the underlying retinal microcircuits. Upon collecting inputs across their respective receptive field, RGCs perform further analysis (e.g., summation, subtraction, weighting) before they generate the final output spike train, which itself is characterized by multiple different features, such as the number of spikes, the inter-spike intervals, response delay, and the rundown time (transience) of the response. These specific kinetic features are essential for target postsynaptic neurons in the brain in order to effectively decode and interpret signals, thereby forming visual perception. We review recent knowledge regarding circuit elements of the mammalian retina that participate in shaping RGC response transience for optimal visual signaling.
Keywords: amacrine cell; bipolar cell; ganglion cell; ganglion cell layer; inner plexiform layer; outer plexiform layer; parallel signaling; photoreceptor; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Dunn F.A., Wong R.O.L., Sidhu S.K., Weavil J.C., Venturelli M., Garten R.S., Rossman M.J., Richardson R.S., Gmelch B.S., Morgan D.E., et al. Wiring patterns in the mouse retina: Collecting evidence across the connectome, physiology and light microscopy. J. Physiol. 2014;592:4809–4823. doi: 10.1113/jphysiol.2014.277228. - DOI - PMC - PubMed
-
- Shekhar K., Lapan S.W., Whitney I.E., Tran N.M., Macosko E.Z., Kowalczyk M., Adiconis X., Levin J., Nemesh J., Goldman M., et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell. 2016;166:1308–1323.e30. doi: 10.1016/j.cell.2016.07.054. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 2019-2.1.7-ERANET-2021-00018/ERA-NET COFUND
- 2017-1.2.1.-NKP-2017/National Research, Development and Innovation Office
- OTKA NN128293/National Research, Development and Innovation Office
- TKP2020 IKA-07 National Excellence Program/European Union and the State of Hungary, co-financed by the European Social Fund
- ÚNKP-20-3-I-PTE-472/Ministry of Human Capacities
LinkOut - more resources
Full Text Sources

