Hsp70 in Redox Homeostasis
- PMID: 35269451
- PMCID: PMC8909019
- DOI: 10.3390/cells11050829
Hsp70 in Redox Homeostasis
Abstract
Cellular redox homeostasis is precisely balanced by generation and elimination of reactive oxygen species (ROS). ROS are not only capable of causing oxidation of proteins, lipids and DNA to damage cells but can also act as signaling molecules to modulate transcription factors and epigenetic pathways that determine cell survival and death. Hsp70 proteins are central hubs for proteostasis and are important factors to ameliorate damage from different kinds of stress including oxidative stress. Hsp70 members often participate in different cellular signaling pathways via their clients and cochaperones. ROS can directly cause oxidative cysteine modifications of Hsp70 members to alter their structure and chaperone activity, resulting in changes in the interactions between Hsp70 and their clients or cochaperones, which can then transfer redox signals to Hsp70-related signaling pathways. On the other hand, ROS also activate some redox-related signaling pathways to indirectly modulate Hsp70 activity and expression. Post-translational modifications including phosphorylation together with elevated Hsp70 expression can expand the capacity of Hsp70 to deal with ROS-damaged proteins and support antioxidant enzymes. Knowledge about the response and role of Hsp70 in redox homeostasis will facilitate our understanding of the cellular knock-on effects of inhibitors targeting Hsp70 and the mechanisms of redox-related diseases and aging.
Keywords: Hsp70; ROS; cysteine modifications; glutathionylation; oxidative stress; redox homeostasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

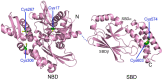
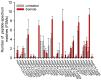

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

