MiR-30a-5p Alters Epidermal Terminal Differentiation during Aging by Regulating BNIP3L/NIX-Dependent Mitophagy
- PMID: 35269458
- PMCID: PMC8909909
- DOI: 10.3390/cells11050836
MiR-30a-5p Alters Epidermal Terminal Differentiation during Aging by Regulating BNIP3L/NIX-Dependent Mitophagy
Abstract
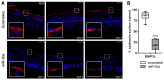
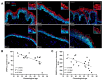
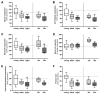
Chronological aging is characterized by an alteration in the genes' regulatory network. In human skin, epidermal keratinocytes fail to differentiate properly with aging, leading to the weakening of the epidermal function. MiR-30a is particularly overexpressed with epidermal aging, but the downstream molecular mechanisms are still uncovered. The aim of this study was to decipher the effects of miR-30a overexpression in the human epidermis, with a focus on keratinocyte differentiation. We formally identified the mitophagy receptor BNIP3L as a direct target of miR-30a. Using a 3D organotypic model of reconstructed human epidermis overexpressing miR-30a, we observed a strong reduction in BNIP3L expression in the granular layer. In human epidermal sections of skin biopsies from donors of different ages, we observed a similar pattern of BNIP3L decreasing with aging. Moreover, human primary keratinocytes undergoing differentiation in vitro also showed a decreased expression of BNIP3L with age, together with a retention of mitochondria. Moreover, aging is associated with altered mitochondrial metabolism in primary keratinocytes, including decreased ATP-linked respiration. Thus, miR-30a is a negative regulator of programmed mitophagy during keratinocytes terminal differentiation, impairing epidermal homeostasis with aging.
Keywords: BNIP3L; aging; keratinocyte; miR-30a; mitochondria; mitophagy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chevalier F.P., Rorteau J., Lamartine J. MicroRNAs in the Functional Defects of Skin Aging. In: Tutar L., Aras S., Tutar E., editors. Non-Coding RNAs. IntechOpen; London, UK: 2020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

