Immunoproteasome Activity in Chronic Lymphocytic Leukemia as a Target of the Immunoproteasome-Selective Inhibitors
- PMID: 35269460
- PMCID: PMC8909520
- DOI: 10.3390/cells11050838
Immunoproteasome Activity in Chronic Lymphocytic Leukemia as a Target of the Immunoproteasome-Selective Inhibitors
Abstract
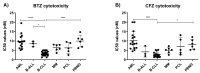
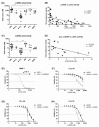
Targeting proteasome with proteasome inhibitors (PIs) is an approved treatment strategy in multiple myeloma that has also been explored pre-clinically and clinically in other hematological malignancies. The approved PIs target both the constitutive and the immunoproteasome, the latter being present predominantly in cells of lymphoid origin. Therapeutic targeting of the immunoproteasome in cells with sole immunoproteasome activity may be selectively cytotoxic in malignant cells, while sparing the non-lymphoid tissues from the on-target PIs toxicity. Using activity-based probes to assess the proteasome activity profile and correlating it with the cytotoxicity assays, we identified B-cell chronic lymphocytic leukemia (B-CLL) to express predominantly immunoproteasome activity, which is associated with high sensitivity to approved proteasome inhibitors and, more importantly, to the immunoproteasome selective inhibitors LU005i and LU035i, targeting all immunoproteasome active subunits or only the immunoproteasome β5i, respectively. At the same time, LU102, a proteasome β2 inhibitor, sensitized B-CLL or immunoproteasome inhibitor-inherently resistant primary cells of acute myeloid leukemia, B-cell acute lymphoblastic leukemia, multiple myeloma and plasma cell leukemia to low doses of LU035i. The immunoproteasome thus represents a novel therapeutic target, which warrants further testing with clinical stage immunoproteasome inhibitors in monotherapy or in combinations.
Keywords: LU005i; LU035i; activity-based probes; acute myeloid leukemia; chronic lymphocytic leukemia; immunoproteasome; multiple myeloma; plasma cell leukemia; proteasome activity; proteasome inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
High Immunoproteasome Activity and sXBP1 in Pediatric Precursor B-ALL Predicts Sensitivity towards Proteasome Inhibitors.Cells. 2021 Oct 22;10(11):2853. doi: 10.3390/cells10112853. Cells. 2021. PMID: 34831075 Free PMC article.
-
Immunoproteasome-selective and non-selective inhibitors: A promising approach for the treatment of multiple myeloma.Pharmacol Ther. 2018 Feb;182:176-192. doi: 10.1016/j.pharmthera.2017.09.001. Epub 2017 Sep 11. Pharmacol Ther. 2018. PMID: 28911826 Review.
-
An inhibitor of proteasome β2 sites sensitizes myeloma cells to immunoproteasome inhibitors.Blood Adv. 2018 Oct 9;2(19):2443-2451. doi: 10.1182/bloodadvances.2018016360. Blood Adv. 2018. PMID: 30266819 Free PMC article.
-
Proteasome Inhibition in Multiple Myeloma: Head-to-Head Comparison of Currently Available Proteasome Inhibitors.Cell Chem Biol. 2019 Mar 21;26(3):340-351.e3. doi: 10.1016/j.chembiol.2018.11.007. Epub 2019 Jan 3. Cell Chem Biol. 2019. PMID: 30612952
-
The immunoproteasome as a target in hematologic malignancies.Semin Hematol. 2012 Jul;49(3):258-62. doi: 10.1053/j.seminhematol.2012.04.003. Semin Hematol. 2012. PMID: 22726549 Free PMC article. Review.
Cited by
-
Immunoproteasome acted as immunotherapy 'coffee companion' in advanced carcinoma therapy.Front Immunol. 2024 Aug 30;15:1464267. doi: 10.3389/fimmu.2024.1464267. eCollection 2024. Front Immunol. 2024. PMID: 39281672 Free PMC article. Review.
-
From oncogenesis to prognosis: the roles of the immunoproteasome in cancer.Front Immunol. 2025 Jul 8;16:1603816. doi: 10.3389/fimmu.2025.1603816. eCollection 2025. Front Immunol. 2025. PMID: 40698074 Free PMC article. Review.
-
The dichotomous role of immunoproteasome in cancer: Friend or foe?Acta Pharm Sin B. 2023 May;13(5):1976-1989. doi: 10.1016/j.apsb.2022.11.005. Epub 2022 Nov 5. Acta Pharm Sin B. 2023. PMID: 37250147 Free PMC article. Review.
References
-
- Chim C.S., Kumar S.K., Orlowski R.Z., Cook G., Richardson P.G., Gertz M.A., Giralt S., Mateos M.V., Leleu X., Anderson K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–262. doi: 10.1038/leu.2017.329. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

