High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases
- PMID: 35269471
- PMCID: PMC8909428
- DOI: 10.3390/cells11050849
High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases
Abstract
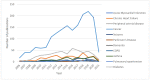
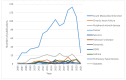
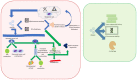
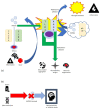
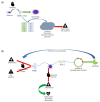
In the early 1970s, a group of non-histone nuclear proteins with high electrophoretic mobility was discovered and named high-mobility group (HMG) proteins. High-mobility group box 1 (HMGB1) is the most studied HMG protein that detects and coordinates cellular stress response. The biological function of HMGB1 depends on its subcellular localization and expression. It plays a critical role in the nucleus and cytoplasm as DNA chaperone, chromosome gatekeeper, autophagy maintainer, and protector from apoptotic cell death. HMGB1 also functions as an extracellular alarmin acting as a damage-associated molecular pattern molecule (DAMP). Recent findings describe HMGB1 as a sophisticated signal of danger, with a pleiotropic function, which is useful as a clinical biomarker for several disorders. HMGB1 has emerged as a mediator in acute and chronic inflammation. Furthermore, HMGB1 targeting can induce beneficial effects on oxidative stress related diseases. This review focus on HMGB1 redox status, localization, mechanisms of release, binding with receptors, and its activities in different oxidative stress-related chronic diseases. Since a growing number of reports show the key role of HMGB1 in socially relevant pathological conditions, to our knowledge, for the first time, here we analyze the scientific literature, evaluating the number of publications focusing on HMGB1 in humans and animal models, per year, from 2006 to 2021 and the number of records published, yearly, per disease and category (studies on humans and animal models).
Keywords: cancer; cardiovascular diseases; damage-associated molecular pattern molecules; diabetes; high-mobility group box 1; inflammation; neurological diseases; oxidative stress related chronic diseases; respiratory diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

