Vesicular MicroRNA as Potential Biomarkers of Viral Rebound
- PMID: 35269481
- PMCID: PMC8909274
- DOI: 10.3390/cells11050859
Vesicular MicroRNA as Potential Biomarkers of Viral Rebound
Abstract
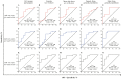
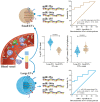
Changes in the cellular microRNA (miRNA) expression profile in response to HIV infection, replication or latency have been reported. Nevertheless, little is known concerning the abundance of miRNA in extracellular vesicles (EVs). In the search for a reliable predictor of viral rebound, we quantified the amount of miR-29a, miR-146a, and miR-155 in two types of plasma extracellular vesicles. Venous blood was collected from 235 ART-treated and ART-naive persons living with HIV (85 with ongoing viral replication, ≥20 copies/mL) and 60 HIV-negative participants at five HIV testing or treatment centers in Burkina Faso. Large and small plasma EVs were purified and counted, and mature miRNA miR-29a, miR-146a, and miR-155 were measured by RT-qPCR. Diagnostic performance of miRNA levels in large and small EVs was evaluated by a receiver operating characteristic curve analysis. The median duration of HIV infection was 36 months (IQR 14-117). The median duration of ART was 34 months (IQR 13-85). The virus was undetectable in 63.8% of these persons. In the others, viral load ranged from 108 to 33,978 copies/mL (median = 30,032). Large EVs were more abundant in viremic participants than aviremic. All three miRNAs were significantly more abundant in small EVs in persons with detectable HIV RNA, and their expression levels in copies per vesicle were a more reliable indicator of viral replication in ART-treated patients with low viremia (20-1000 copies/mL). HIV replication increased the production of large EVs more than small EVs. Combined with viral load measurement, quantifying EV-associated miRNA abundance relative to the number of vesicles provides a more reliable marker of the viral status. The expression level as copies per small vesicle could predict the viral rebound in ART-treated patients with undetectable viral loads.
Keywords: HIV-1; biomarker; extracellular vesicles; miR-146a; miR-155; miR-29a; microRNA; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
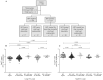
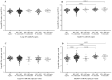

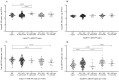
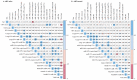


References
-
- Aamer H.A., McClure J., Ko D., Maenza J., Collier A.C., Coombs R.W., Mullins J.I., Frenkel L.M. Cells producing residual viremia during antiretroviral treatment appear to contribute to rebound viremia following interruption of treatment. PLoS Pathog. 2020;16:e1008791. doi: 10.1371/journal.ppat.1008791. - DOI - PMC - PubMed
-
- Dinoso J.B., Kim S.Y., Wiegand A.M., Palmer S.E., Gange S.J., Cranmer L., O’Shea A., Callender M., Spivak A., Brennan T., et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. - DOI - PMC - PubMed
-
- Vandenhende M.A., Perrier A., Bonnet F., Lazaro E., Cazanave C., Reigadas S., Chêne G., Morlat P. Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study) Antivir. Ther. 2015;20:655–660. doi: 10.3851/IMP2949. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

