Molecular Mechanisms of Alveolar Epithelial Stem Cell Senescence and Senescence-Associated Differentiation Disorders in Pulmonary Fibrosis
- PMID: 35269498
- PMCID: PMC8909789
- DOI: 10.3390/cells11050877
Molecular Mechanisms of Alveolar Epithelial Stem Cell Senescence and Senescence-Associated Differentiation Disorders in Pulmonary Fibrosis
Abstract
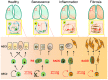
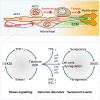
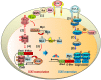
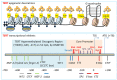
Pulmonary senescence is accelerated by unresolved DNA damage response, underpinning susceptibility to pulmonary fibrosis. Recently it was reported that the SARS-Cov-2 viral infection induces acute pulmonary epithelial senescence followed by fibrosis, although the mechanism remains unclear. Here, we examine roles of alveolar epithelial stem cell senescence and senescence-associated differentiation disorders in pulmonary fibrosis, exploring the mechanisms mediating and preventing pulmonary fibrogenic crisis. Notably, the TGF-β signalling pathway mediates alveolar epithelial stem cell senescence by mechanisms involving suppression of the telomerase reverse transcriptase gene in pulmonary fibrosis. Alternatively, telomere uncapping caused by stress-induced telomeric shelterin protein TPP1 degradation mediates DNA damage response, pulmonary senescence and fibrosis. However, targeted intervention of cellular senescence disrupts pulmonary remodelling and fibrosis by clearing senescent cells using senolytics or preventing senescence using telomere dysfunction inhibitor (TELODIN). Studies indicate that the development of senescence-associated differentiation disorders is reprogrammable and reversible by inhibiting stem cell replicative senescence in pulmonary fibrosis, providing a framework for targeted intervention of the molecular mechanisms of alveolar stem cell senescence and pulmonary fibrosis. Abbreviations: DPS, developmental programmed senescence; IPF, idiopathic pulmonary fibrosis; OIS, oncogene-induced replicative senescence; SADD, senescence-associated differentiation disorder; SALI, senescence-associated low-grade inflammation; SIPS, stress-induced premature senescence; TERC, telomerase RNA component; TERT, telomerase reverse transcriptase; TIFs, telomere dysfunction-induced foci; TIS, therapy-induced senescence; VIS, virus-induced senescence.
Keywords: COVID-19; DNA damage response; TGF-β signalling; pulmonary fibrosis; replicative senescence; telomerase and telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pulmonary Alveolar Stem Cell Senescence, Apoptosis, and Differentiation by p53-Dependent and -Independent Mechanisms in Telomerase-Deficient Mice.Cells. 2021 Oct 26;10(11):2892. doi: 10.3390/cells10112892. Cells. 2021. PMID: 34831112 Free PMC article.
-
FBW7 Mediates Senescence and Pulmonary Fibrosis through Telomere Uncapping.Cell Metab. 2020 Nov 3;32(5):860-877.e9. doi: 10.1016/j.cmet.2020.10.004. Epub 2020 Oct 20. Cell Metab. 2020. PMID: 33086033
-
Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence.J Biol Chem. 2019 May 31;294(22):8861-8871. doi: 10.1074/jbc.RA118.006615. Epub 2019 Apr 18. J Biol Chem. 2019. PMID: 31000627 Free PMC article.
-
Tissue formation and tissue engineering through host cell recruitment or a potential injectable cell-based biocomposite with replicative potential: Molecular mechanisms controlling cellular senescence and the involvement of controlled transient telomerase activation therapies.J Biomed Mater Res A. 2015 Dec;103(12):3993-4023. doi: 10.1002/jbm.a.35515. Epub 2015 Aug 14. J Biomed Mater Res A. 2015. PMID: 26034007 Review.
-
Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis.Dis Model Mech. 2013 Jan;6(1):9-17. doi: 10.1242/dmm.010736. Dis Model Mech. 2013. PMID: 23268535 Free PMC article. Review.
Cited by
-
Controversies and Recent Advances in Senescence and Aging.Cells. 2023 Mar 15;12(6):902. doi: 10.3390/cells12060902. Cells. 2023. PMID: 36980243 Free PMC article.
-
Short airway telomeres are associated with primary graft dysfunction and chronic lung allograft dysfunction.J Heart Lung Transplant. 2023 Dec;42(12):1700-1709. doi: 10.1016/j.healun.2023.08.018. Epub 2023 Aug 28. J Heart Lung Transplant. 2023. PMID: 37648073 Free PMC article.
-
Human umbilical cord mesenchymal stem cell-derived microvesicles alleviate pulmonary fibrosis by inhibiting monocyte‒macrophage migration through ERK1/2 signaling-mediated suppression of CCL2 expression.Stem Cell Res Ther. 2025 Mar 24;16(1):145. doi: 10.1186/s13287-025-04266-w. Stem Cell Res Ther. 2025. PMID: 40128840 Free PMC article.
-
Cellular Senescence: A Troy Horse in Pulmonary Fibrosis.Int J Mol Sci. 2023 Nov 16;24(22):16410. doi: 10.3390/ijms242216410. Int J Mol Sci. 2023. PMID: 38003600 Free PMC article. Review.
-
Modulating in vitro lung fibroblast activation via senolysis of senescent human alveolar epithelial cells.Aging (Albany NY). 2024 Jun 29;16(13):10694-10723. doi: 10.18632/aging.205994. Epub 2024 Jun 29. Aging (Albany NY). 2024. PMID: 38976646 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

