PTH-Induced Bone Regeneration and Vascular Modulation Are Both Dependent on Endothelial Signaling
- PMID: 35269519
- PMCID: PMC8909576
- DOI: 10.3390/cells11050897
PTH-Induced Bone Regeneration and Vascular Modulation Are Both Dependent on Endothelial Signaling
Abstract
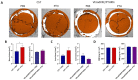
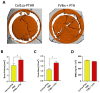
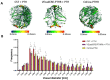
The use of a bone allograft presents a promising approach for healing nonunion fractures. We have previously reported that parathyroid hormone (PTH) therapy induced allograft integration while modulating angiogenesis at the allograft proximity. Here, we hypothesize that PTH-induced vascular modulation and the osteogenic effect of PTH are both dependent on endothelial PTH receptor-1 (PTHR1) signaling. To evaluate our hypothesis, we used multiple transgenic mouse lines, and their wild-type counterparts as a control. In addition to endothelial-specific PTHR1 knock-out mice, we used mice in which PTHR1 was engineered to be constitutively active in collagen-1α+ osteoblasts, to assess the effect of PTH signaling activation exclusively in osteoprogenitors. To characterize resident cell recruitment and osteogenic activity, mice in which the Luciferase reporter gene is expressed under the Osteocalcin promoter (Oc-Luc) were used. Mice were implanted with calvarial allografts and treated with either PTH or PBS. A micro-computed tomography-based structural analysis indicated that the induction of bone formation by PTH, as observed in wild-type animals, was not maintained when PTHR1 was removed from endothelial cells. Furthermore, the induction of PTH signaling exclusively in osteoblasts resulted in significantly less bone formation compared to systemic PTH treatment, and significantly less osteogenic activity was measured by bioluminescence imaging of the Oc-Luc mice. Deletion of the endothelial PTHR1 significantly decreased the PTH-induced formation of narrow blood vessels, formerly demonstrated in wild-type mice. However, the exclusive activation of PTH signaling in osteoblasts was sufficient to re-establish the observed PTH effect. Collectively, our results show that endothelial PTHR1 signaling plays a key role in PTH-induced osteogenesis and has implications in angiogenesis.
Keywords: allograft; angiogenesis; calvarial bone repair; fracture healing; osteogenesis; parathyroid hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
PTH promotes allograft integration in a calvarial bone defect.Mol Pharm. 2013 Dec 2;10(12):4462-71. doi: 10.1021/mp400292p. Epub 2013 Nov 8. Mol Pharm. 2013. PMID: 24131143 Free PMC article.
-
Parathyroid hormone induces bone formation in phosphorylation-deficient PTHR1 knockin mice.Am J Physiol Endocrinol Metab. 2012 May 1;302(10):E1183-8. doi: 10.1152/ajpendo.00380.2011. Epub 2012 Feb 14. Am J Physiol Endocrinol Metab. 2012. PMID: 22338074 Free PMC article.
-
Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation.J Cell Physiol. 2012 Feb;227(2):408-15. doi: 10.1002/jcp.22986. J Cell Physiol. 2012. PMID: 21866553 Free PMC article.
-
Histological functions of parathyroid hormone on bone formation and bone blood vessels.J Oral Biosci. 2022 Sep;64(3):279-286. doi: 10.1016/j.job.2022.08.002. Epub 2022 Aug 14. J Oral Biosci. 2022. PMID: 35977651 Review.
-
Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies.Biochem Pharmacol. 2013 May 15;85(10):1417-23. doi: 10.1016/j.bcp.2013.03.002. Epub 2013 Mar 13. Biochem Pharmacol. 2013. PMID: 23500550 Review.
Cited by
-
Calcium Phosphates: A Key to Next-Generation In Vitro Bone Modeling.Adv Healthc Mater. 2024 Nov;13(29):e2401307. doi: 10.1002/adhm.202401307. Epub 2024 Aug 23. Adv Healthc Mater. 2024. PMID: 39175382 Free PMC article. Review.
-
Teriparatide as a non-surgical salvage therapy for prolonged humerus fracture nonunion: A case report and literature review.World J Orthop. 2025 Jan 18;16(1):101656. doi: 10.5312/wjo.v16.i1.101656. eCollection 2025 Jan 18. World J Orthop. 2025. PMID: 39850036 Free PMC article.
-
Basigin links altered skeletal stem cell lineage dynamics with glucocorticoid-induced bone loss and impaired angiogenesis.Nat Commun. 2025 Aug 15;16(1):7606. doi: 10.1038/s41467-025-62881-w. Nat Commun. 2025. PMID: 40817087 Free PMC article.
-
Similarities and Differences of Vascular Calcification in Diabetes and Chronic Kidney Disease.Diabetes Metab Syndr Obes. 2024 Jan 10;17:165-192. doi: 10.2147/DMSO.S438618. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38222032 Free PMC article. Review.
-
Short-Term Administration of Parathyroid Hormone Improves Wound Healing Around Implants in an Osteoporotic Rat Model.J Clin Med. 2025 Jun 1;14(11):3900. doi: 10.3390/jcm14113900. J Clin Med. 2025. PMID: 40507663 Free PMC article.
References
-
- Curtis E.M., Van der Velde R., Moon R.J., Van den Bergh J.P., Geusens P., De Vries F., Van Staa T.P., Cooper C., Harvey N.C. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

