Direct Interaction of Mitochondrial Cytochrome c Oxidase with Thyroid Hormones: Evidence for Two Binding Sites
- PMID: 35269529
- PMCID: PMC8909594
- DOI: 10.3390/cells11050908
Direct Interaction of Mitochondrial Cytochrome c Oxidase with Thyroid Hormones: Evidence for Two Binding Sites
Abstract
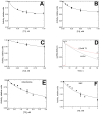
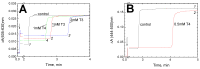
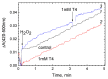
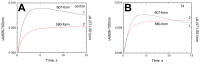
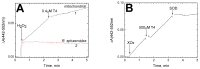
Thyroid hormones regulate tissue metabolism to establish an energy balance in the cell, in particular, by affecting oxidative phosphorylation. Their long-term impact is mainly associated with changes in gene expression, while the short-term effects may differ in their mechanisms. Our work was devoted to studying the short-term effects of hormones T2, T3 and T4 on mitochondrial cytochrome c oxidase (CcO) mediated by direct contact with the enzyme. The data obtained indicate the existence of two separate sites of CcO interaction with thyroid hormones, differing in their location, affinity and specificity to hormone binding. First, we show that T3 and T4 but not T2 inhibit the oxidase activity of CcO in solution and on membrane preparations with Ki ≈ 100-200 μM. In solution, T3 and T4 compete in a 1:1 ratio with the detergent dodecyl-maltoside to bind to the enzyme. The peroxidase and catalase partial activities of CcO are not sensitive to hormones, but electron transfer from heme a to the oxidized binuclear center is affected. We believe that T3 and T4 could be ligands of the bile acid-binding site found in the 3D structure of CcO by Ferguson-Miller's group, and hormone-induced inhibition is associated with dysfunction of the K-proton channel. A possible role of this interaction in the physiological regulation of the enzyme is discussed. Second, we find that T2, T3, and T4 inhibit superoxide generation by oxidized CcO in the presence of excess H2O2. Inhibition is characterized by Ki values of 0.3-5 μM and apparently affects the formation of O2●- at the protein surface. The second binding site for thyroid hormones presumably coincides with the point of tight T2 binding on the Va subunit described in the literature.
Keywords: bile acid-binding site; cytochrome oxidase; regulation; steroid hormones; superoxide generation; thyroid hormones.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

