Dynamics of Endothelial Engagement and Filopodia Formation in Complex 3D Microscaffolds
- PMID: 35269558
- PMCID: PMC8910162
- DOI: 10.3390/ijms23052415
Dynamics of Endothelial Engagement and Filopodia Formation in Complex 3D Microscaffolds
Abstract
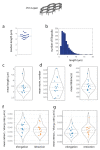
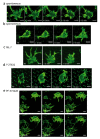
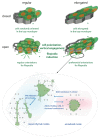
The understanding of endothelium-extracellular matrix interactions during the initiation of new blood vessels is of great medical importance; however, the mechanobiological principles governing endothelial protrusive behaviours in 3D microtopographies remain imperfectly understood. In blood capillaries submitted to angiogenic factors (such as vascular endothelial growth factor, VEGF), endothelial cells can transiently transdifferentiate in filopodia-rich cells, named tip cells, from which angiogenesis processes are locally initiated. This protrusive state based on filopodia dynamics contrasts with the lamellipodia-based endothelial cell migration on 2D substrates. Using two-photon polymerization, we generated 3D microstructures triggering endothelial phenotypes evocative of tip cell behaviour. Hexagonal lattices on pillars ("open"), but not "closed" hexagonal lattices, induced engagement from the endothelial monolayer with the generation of numerous filopodia. The development of image analysis tools for filopodia tracking allowed to probe the influence of the microtopography (pore size, regular vs. elongated structures, role of the pillars) on orientations, engagement and filopodia dynamics, and to identify MLCK (myosin light-chain kinase) as a key player for filopodia-based protrusive mode. Importantly, these events occurred independently of VEGF treatment, suggesting that the observed phenotype was induced through microtopography. These microstructures are proposed as a model research tool for understanding endothelial cell behaviour in 3D fibrillary networks.
Keywords: angiogenesis; contractility; endothelial cells; filopodia; mechanotransduction; microtopography; two-photon polymerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures











Similar articles
-
Filopodia are dispensable for endothelial tip cell guidance.Development. 2013 Oct;140(19):4031-40. doi: 10.1242/dev.097352. Development. 2013. PMID: 24046319
-
NRP1 Regulates CDC42 Activation to Promote Filopodia Formation in Endothelial Tip Cells.Cell Rep. 2015 Jun 16;11(10):1577-90. doi: 10.1016/j.celrep.2015.05.018. Epub 2015 Jun 4. Cell Rep. 2015. PMID: 26051942 Free PMC article.
-
Endothelial tip-cell position, filopodia formation and biomechanics require BMPR2 expression and signaling.Commun Biol. 2025 Jan 8;8(1):21. doi: 10.1038/s42003-024-07431-8. Commun Biol. 2025. PMID: 39779836 Free PMC article.
-
Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way.Arterioscler Thromb Vasc Biol. 2009 May;29(5):639-49. doi: 10.1161/ATVBAHA.109.185165. Epub 2009 Mar 5. Arterioscler Thromb Vasc Biol. 2009. PMID: 19265031 Review.
-
Filopodia and focal adhesions: An integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis.Dev Biol. 2019 Jul 1;451(1):86-95. doi: 10.1016/j.ydbio.2018.08.015. Epub 2018 Sep 5. Dev Biol. 2019. PMID: 30193787 Free PMC article. Review.
Cited by
-
Endothelial cells signaling and patterning under hypoxia: a mechanistic integrative computational model including the Notch-Dll4 pathway.Front Physiol. 2024 Feb 22;15:1351753. doi: 10.3389/fphys.2024.1351753. eCollection 2024. Front Physiol. 2024. PMID: 38455844 Free PMC article.
-
Cytoskeletal regulation on polycaprolactone/graphene porous scaffolds for bone tissue engineering.Sci Rep. 2024 Nov 23;14(1):29062. doi: 10.1038/s41598-024-80467-2. Sci Rep. 2024. PMID: 39580502 Free PMC article.
-
Synergistic effect of hierarchical topographic structure on 3D-printed Titanium scaffold for enhanced coupling of osteogenesis and angiogenesis.Mater Today Bio. 2023 Nov 25;23:100866. doi: 10.1016/j.mtbio.2023.100866. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38149019 Free PMC article.
References
-
- Karina Kulangaraa K.W.L. Substrate topography shapes cell function. Soft Matter. 2009;5:4072–4076. doi: 10.1039/b910132m. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

