Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults
- PMID: 35269565
- PMCID: PMC8910114
- DOI: 10.3390/ijms23052421
Molecular Mechanisms and Physiological Changes behind Benign Tracheal and Subglottic Stenosis in Adults
Abstract
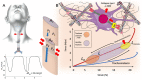
Laryngotracheal stenosis (LTS) is a complex and heterogeneous disease whose pathogenesis remains unclear. LTS is considered to be the result of aberrant wound-healing process that leads to fibrotic scarring, originating from different aetiology. Although iatrogenic aetiology is the main cause of subglottic or tracheal stenosis, also autoimmune and infectious diseases may be involved in causing LTS. Furthermore, fibrotic obstruction in the anatomic region under the glottis can also be diagnosed without apparent aetiology after a comprehensive workup; in this case, the pathological process is called idiopathic subglottic stenosis (iSGS). So far, the laryngotracheal scar resulting from airway injury due to different diseases was considered as inert tissue requiring surgical removal to restore airway patency. However, this assumption has recently been revised by regarding the tracheal scarring process as a fibroinflammatory event due to immunological alteration, similar to other fibrotic diseases. Recent acquisitions suggest that different factors, such as growth factors, cytokines, altered fibroblast function and genetic susceptibility, can all interact in a complex way leading to aberrant and fibrotic wound healing after an insult that acts as a trigger. However, also physiological derangement due to LTS could play a role in promoting dysregulated response to laryngo-tracheal mucosal injury, through biomechanical stress and mechanotransduction activation. The aim of this narrative review is to present the state-of-the-art knowledge regarding molecular mechanisms, as well as mechanical and physio-pathological features behind LTS.
Keywords: granulomatosis with polyangiitis; relapsing polychondritis; subglottic stenosis; tracheal stenosis; tracheostomy; web-like stenosis.
Conflict of interest statement
The authors have no competing interests with any organization or entity with a financial interest in competition with the subject, matter, or materials discussed in the manuscript.
Figures

References
-
- Wong J.W., Gallant-Behm C., Wiebe C., Mak K., Hart D.A., Larjava H., Häkkinen L. Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red Duroc pig model and humans. Wound Repair Regen. 2009;17:717–729. doi: 10.1111/j.1524-475X.2009.00531.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

