A Review of Alpha-1 Antitrypsin Binding Partners for Immune Regulation and Potential Therapeutic Application
- PMID: 35269582
- PMCID: PMC8910375
- DOI: 10.3390/ijms23052441
A Review of Alpha-1 Antitrypsin Binding Partners for Immune Regulation and Potential Therapeutic Application
Abstract
Alpha-1 antitrypsin (AAT) is the canonical serine protease inhibitor of neutrophil-derived proteases and can modulate innate immune mechanisms through its anti-inflammatory activities mediated by a broad spectrum of protein, cytokine, and cell surface interactions. AAT contains a reactive methionine residue that is critical for its protease-specific binding capacity, whereby AAT entraps the protease on cleavage of its reactive centre loop, neutralises its activity by key changes in its tertiary structure, and permits removal of the AAT-protease complex from the circulation. Recently, however, the immunomodulatory role of AAT has come increasingly to the fore with several prominent studies focused on lipid or protein-protein interactions that are predominantly mediated through electrostatic, glycan, or hydrophobic potential binding sites. The aim of this review was to investigate the spectrum of AAT molecular interactions, with newer studies supporting a potential therapeutic paradigm for AAT augmentation therapy in disorders in which a chronic immune response is strongly linked.
Keywords: alpha-1 antitrypsin; alpha-1 antitrypsin deficiency; complement C3; coronavirus disease 2019 (COVID-19); cytokines; interleukin-6; proteases.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish the results.
Figures
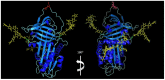
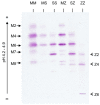
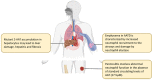

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

