The Ism between Endothelial Cilia and Endothelial Nanotubules Is an Evolving Concept in the Genesis of the BBB
- PMID: 35269595
- PMCID: PMC8910322
- DOI: 10.3390/ijms23052457
The Ism between Endothelial Cilia and Endothelial Nanotubules Is an Evolving Concept in the Genesis of the BBB
Abstract
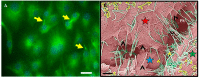
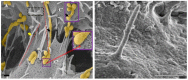
The blood-brain barrier (BBB) is fundamental in maintaining central nervous system (CNS) homeostasis by regulating the chemical environment of the underlying brain parenchyma. Brain endothelial cells (BECs) constitute the anatomical and functional basis of the BBB. Communication between adjacent BECs is critical for establishing BBB integrity, and knowledge of its nanoscopic landscape will contribute to our understanding of how juxtaposed zones of tight-junction protein interactions between BECs are aligned. The review discusses and critiques types of nanostructures contributing to the process of BBB genesis. We further critically evaluate earlier findings in light of novel high-resolution electron microscopy descriptions of nanoscopic tubules. One such phenotypic structure is BEC cytoplasmic projections, which, early in the literature, is postulated as brain capillary endothelial cilia, and is evaluated and compared to the recently discovered nanotubules (NTs) formed in the paracellular spaces between BECs during barrier-genesis. The review attempts to elucidate a myriad of unique topographical ultrastructures that have been reported to be associated with the development of the BBB, viz., structures ranging from cilia to BEC tunneling nanotubules (TUNTs) and BEC tethering nanotubules (TENTs).
Keywords: BBB; brain endothelium; cilium; cytoplasmic projections; tethering nanotubules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
High-Resolution Insights Into the in vitro Developing Blood-Brain Barrier: Novel Morphological Features of Endothelial Nanotube Function.Front Neuroanat. 2021 Jun 25;15:661065. doi: 10.3389/fnana.2021.661065. eCollection 2021. Front Neuroanat. 2021. PMID: 34248507 Free PMC article.
-
Exosomes form tunneling nanotubes (TUNTs) in the blood-brain barrier: a nano-anatomical perspective of barrier genesis.Front Mol Neurosci. 2022 Sep 20;15:938315. doi: 10.3389/fnmol.2022.938315. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36204136 Free PMC article.
-
The Role of Cytoskeletal Proteins in the Formation of a Functional In Vitro Blood-Brain Barrier Model.Int J Mol Sci. 2022 Jan 11;23(2):742. doi: 10.3390/ijms23020742. Int J Mol Sci. 2022. PMID: 35054928 Free PMC article.
-
Methods used for the measurement of blood-brain barrier integrity.Metab Brain Dis. 2021 Jun;36(5):723-735. doi: 10.1007/s11011-021-00694-8. Epub 2021 Feb 26. Metab Brain Dis. 2021. PMID: 33635479 Review.
-
Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection.Am J Physiol Cell Physiol. 2018 Sep 1;315(3):C343-C356. doi: 10.1152/ajpcell.00095.2018. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949404 Free PMC article. Review.
Cited by
-
Islet cilia and glucose homeostasis.Front Cell Dev Biol. 2022 Dec 1;10:1082193. doi: 10.3389/fcell.2022.1082193. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36531945 Free PMC article. Review.
-
MicroRNA-126 as an endogenous molecule for hypoxic/ischemic tolerance in neuroprotection.Neurol Sci. 2025 Aug 23. doi: 10.1007/s10072-025-08413-2. Online ahead of print. Neurol Sci. 2025. PMID: 40846814 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

