Impact of Two Neuronal Sigma-1 Receptor Modulators, PRE084 and DMT, on Neurogenesis and Neuroinflammation in an Aβ1-42-Injected, Wild-Type Mouse Model of AD
- PMID: 35269657
- PMCID: PMC8910266
- DOI: 10.3390/ijms23052514
Impact of Two Neuronal Sigma-1 Receptor Modulators, PRE084 and DMT, on Neurogenesis and Neuroinflammation in an Aβ1-42-Injected, Wild-Type Mouse Model of AD
Abstract
Alzheimer's disease (AD) is the most common form of dementia characterized by cognitive dysfunctions. Pharmacological interventions to slow the progression of AD are intensively studied. A potential direction targets neuronal sigma-1 receptors (S1Rs). S1R ligands are recognized as promising therapeutic agents that may alleviate symptom severity of AD, possibly via preventing amyloid-β-(Aβ-) induced neurotoxicity on the endoplasmic reticulum stress-associated pathways. Furthermore, S1Rs may also modulate adult neurogenesis, and the impairment of this process is reported to be associated with AD. We aimed to investigate the effects of two S1R agonists, dimethyltryptamine (DMT) and PRE084, in an Aβ-induced in vivo mouse model characterizing neurogenic and anti-neuroinflammatory symptoms of AD, and the modulatory effects of S1R agonists were analyzed by immunohistochemical methods and western blotting. DMT, binding moderately to S1R but with high affinity to 5-HT receptors, negatively influenced neurogenesis, possibly as a result of activating both receptors differently. In contrast, the highly selective S1R agonist PRE084 stimulated hippocampal cell proliferation and differentiation. Regarding neuroinflammation, DMT and PRE084 significantly reduced Aβ1-42-induced astrogliosis, but neither had remarkable effects on microglial activation. In summary, the highly selective S1R agonist PRE084 may be a promising therapeutic agent for AD. Further studies are required to clarify the multifaceted neurogenic and anti-neuroinflammatory roles of these agonists.
Keywords: Alzheimer’s disease; Aβ1–42-induce mouse model; PRE084; dimethyltryptamine; neurogenesis; neuroinflammation; sigma-1 receptor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, nor in the collection, analyses, or interpretation of data, nor in the writing of the manuscript, nor in the decision to publish the results.
Figures
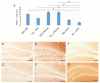

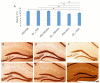
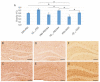
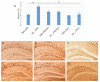
Similar articles
-
N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer's disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk.Alzheimers Res Ther. 2024 May 1;16(1):95. doi: 10.1186/s13195-024-01462-3. Alzheimers Res Ther. 2024. PMID: 38693554 Free PMC article.
-
Social and contextual memory impairments induced by Amyloid-β oligomers are rescued by Sigma-1 receptor activation.Biomed Pharmacother. 2025 Mar;184:117914. doi: 10.1016/j.biopha.2025.117914. Epub 2025 Feb 24. Biomed Pharmacother. 2025. PMID: 39999645
-
The N-Formyl Peptide Receptor 2 (FPR2) Agonist MR-39 Improves Ex Vivo and In Vivo Amyloid Beta (1-42)-Induced Neuroinflammation in Mouse Models of Alzheimer's Disease.Mol Neurobiol. 2021 Dec;58(12):6203-6221. doi: 10.1007/s12035-021-02543-2. Epub 2021 Sep 1. Mol Neurobiol. 2021. PMID: 34468933 Free PMC article.
-
Sigma ligands as potent inhibitors of Aβ and AβOs in neurons and promising therapeutic agents of Alzheimer's disease.Neuropharmacology. 2021 Jun 1;190:108342. doi: 10.1016/j.neuropharm.2020.108342. Epub 2020 Oct 9. Neuropharmacology. 2021. PMID: 33045243 Review.
-
The Sigma-1 Receptor-A Therapeutic Target for the Treatment of ALS?Adv Exp Med Biol. 2017;964:255-265. doi: 10.1007/978-3-319-50174-1_17. Adv Exp Med Biol. 2017. PMID: 28315276 Review.
Cited by
-
Human pluripotent stem cells as a translational toolkit in psychedelic research in vitro.iScience. 2024 Mar 28;27(5):109631. doi: 10.1016/j.isci.2024.109631. eCollection 2024 May 17. iScience. 2024. PMID: 38628967 Free PMC article. Review.
-
Psychoactive substances: novel molecular insights and therapeutic potential for Alzheimer's disease.Transl Neurodegener. 2025 Jul 25;14(1):38. doi: 10.1186/s40035-025-00498-1. Transl Neurodegener. 2025. PMID: 40713680 Free PMC article. Review.
-
The past and present of therapeutic strategy for Alzheimer's diseases: potential for stem cell therapy.Exp Anim. 2023 Aug 7;72(3):285-293. doi: 10.1538/expanim.22-0164. Epub 2023 Mar 6. Exp Anim. 2023. PMID: 36878603 Free PMC article. Review.
-
The Missing Piece? A Case for Microglia's Prominent Role in the Therapeutic Action of Anesthetics, Ketamine, and Psychedelics.Neurochem Res. 2023 Apr;48(4):1129-1166. doi: 10.1007/s11064-022-03772-0. Epub 2022 Nov 3. Neurochem Res. 2023. PMID: 36327017 Review.
-
Targeting Sigma Receptors for the Treatment of Neurodegenerative and Neurodevelopmental Disorders.CNS Drugs. 2023 May;37(5):399-440. doi: 10.1007/s40263-023-01007-6. Epub 2023 May 11. CNS Drugs. 2023. PMID: 37166702 Free PMC article. Review.
References
-
- Alzheimer A., Stelzmann R.A., Schnitzlein H.N., Murtagh F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995;8:429–431. - PubMed
-
- Karlawish J. 2017 Alzheimer’s Disease Facts and Figures. Alzheimer’s Association; Chicago, IL, USA: 2017. p. 88.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

