Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation-A Conceptual Review Summarizing Current Knowledge
- PMID: 35269679
- PMCID: PMC8910683
- DOI: 10.3390/ijms23052536
Mechanisms Underlying Dichotomous Procoagulant COAT Platelet Generation-A Conceptual Review Summarizing Current Knowledge
Abstract
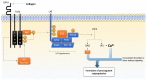
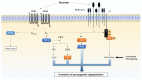
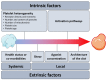
Procoagulant platelets are a subtype of activated platelets that sustains thrombin generation in order to consolidate the clot and stop bleeding. This aspect of platelet activation is gaining more and more recognition and interest. In fact, next to aggregating platelets, procoagulant platelets are key regulators of thrombus formation. Imbalance of both subpopulations can lead to undesired thrombotic or bleeding events. COAT platelets derive from a common pro-aggregatory phenotype in cells capable of accumulating enough cytosolic calcium to trigger specific pathways that mediate the loss of their aggregating properties and the development of new adhesive and procoagulant characteristics. Complex cascades of signaling events are involved and this may explain why an inter-individual variability exists in procoagulant potential. Nowadays, we know the key agonists and mediators underlying the generation of a procoagulant platelet response. However, we still lack insight into the actual mechanisms controlling this dichotomous pattern (i.e., procoagulant versus aggregating phenotype). In this review, we describe the phenotypic characteristics of procoagulant COAT platelets, we detail the current knowledge on the mechanisms of the procoagulant response, and discuss possible drivers of this dichotomous diversification, in particular addressing the impact of the platelet environment during in vivo thrombus formation.
Keywords: COAT; hemostasis; heterogeneity; procoagulant platelets; regulation; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alberio L., Safa O., Clemetson K.J., Esmon C.T., Dale G.L. Surface expression and functional characterization of α-granule factor V in human platelets: Effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–1702. doi: 10.1182/blood.V95.5.1694.005k24_1694_1702. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

