Reorienting Mechanism of Harderoheme in Coproheme Decarboxylase-A Computational Study
- PMID: 35269706
- PMCID: PMC8910490
- DOI: 10.3390/ijms23052564
Reorienting Mechanism of Harderoheme in Coproheme Decarboxylase-A Computational Study
Abstract
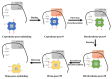
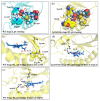
Coproheme decarboxylase (ChdC) is an important enzyme in the coproporphyrin-dependent pathway (CPD) of Gram-positive bacteria that decarboxylates coproheme on two propionates at position 2 and position 4 sequentially to generate heme b by using H2O2 as an oxidant. This work focused on the ChdC from Geobacillus stearothermophilus (GsChdC) to elucidate the mechanism of its sequential two-step decarboxylation of coproheme. The models of GsChdC in a complex with substrate and reaction intermediate were built to investigate the reorienting mechanism of harderoheme. Targeted molecular dynamics simulations on these models validated that harderoheme is able to rotate in the active site of GsChdC with a 19.06-kcal·mol-1 energy barrier after the first step of decarboxylation to bring the propionate at position 4 in proximity of Tyr145 to continue the second decarboxylation step. The harderoheme rotation mechanism is confirmed to be much easier than the release-rebinding mechanism. In the active site of GsChdC, Trp157 and Trp198 comprise a "gate" construction to regulate the clockwise rotation of the harderoheme. Lys149 plays a critical role in the rotation mechanism, which not only keeps the Trp157-Trp198 "gate" from being closed but also guides the propionate at position 4 through the gap between Trp157 and Trp198 through a salt bridge interaction.
Keywords: coproheme decarboxylase; rotation; targeted molecular dynamics simulation; two-step decarboxylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Ruffling drives coproheme decarboxylation by facilitating PCET: a theoretical investigation of ChdC.Phys Chem Chem Phys. 2020 Jul 22;22(28):16117-16124. doi: 10.1039/d0cp02690e. Phys Chem Chem Phys. 2020. PMID: 32638770
-
Tyrosyl Radical-Mediated Sequential Oxidative Decarboxylation of Coproporphyrinogen III through PCET: Theoretical Insights into the Mechanism of Coproheme Decarboxylase ChdC.Inorg Chem. 2021 Sep 6;60(17):13539-13549. doi: 10.1021/acs.inorgchem.1c01864. Epub 2021 Aug 12. Inorg Chem. 2021. PMID: 34382397
-
Decarboxylation involving a ferryl, propionate, and a tyrosyl group in a radical relay yields heme b.J Biol Chem. 2018 Mar 16;293(11):3989-3999. doi: 10.1074/jbc.RA117.000830. Epub 2018 Feb 2. J Biol Chem. 2018. PMID: 29414780 Free PMC article.
-
HemQ: An iron-coproporphyrin oxidative decarboxylase for protoheme synthesis in Firmicutes and Actinobacteria.Arch Biochem Biophys. 2015 May 15;574:27-35. doi: 10.1016/j.abb.2015.02.017. Epub 2015 Feb 21. Arch Biochem Biophys. 2015. PMID: 25711532 Free PMC article. Review.
-
Functionally diverse biotin-dependent enzymes with oxaloacetate decarboxylase activity.Arch Biochem Biophys. 2014 Feb 15;544:75-86. doi: 10.1016/j.abb.2013.10.014. Epub 2013 Oct 30. Arch Biochem Biophys. 2014. PMID: 24184447 Review.
Cited by
-
Relationship between preoperative hemoglobin levels and length of stay in elderly patients with hip fractures: A retrospective cohort study.Medicine (Baltimore). 2024 Jun 21;103(25):e38518. doi: 10.1097/MD.0000000000038518. Medicine (Baltimore). 2024. PMID: 38905374 Free PMC article.
-
The Role of the Hydrogen Bond Network in Maintaining Heme Pocket Stability and Protein Function Specificity of C. diphtheriae Coproheme Decarboxylase.Biomolecules. 2023 Jan 25;13(2):235. doi: 10.3390/biom13020235. Biomolecules. 2023. PMID: 36830604 Free PMC article.
-
Reactivity of Coproheme Decarboxylase with Monovinyl, Monopropionate Deuteroheme.Biomolecules. 2023 Jun 6;13(6):946. doi: 10.3390/biom13060946. Biomolecules. 2023. PMID: 37371526 Free PMC article.
-
Structural aspects of enzymes involved in prokaryotic Gram-positive heme biosynthesis.Comput Struct Biotechnol J. 2023 Jul 24;21:3933-3945. doi: 10.1016/j.csbj.2023.07.024. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37593721 Free PMC article. Review.
-
Scutellarin activates IDH1 to exert antitumor effects in hepatocellular carcinoma progression.Cell Death Dis. 2024 Apr 15;15(4):267. doi: 10.1038/s41419-024-06625-6. Cell Death Dis. 2024. PMID: 38622131 Free PMC article.
References
-
- Gledhill M. The determination of heme b in marine phyto- and bacterioplankton. Mar. Chem. 2007;103:393–403. doi: 10.1016/j.marchem.2006.10.008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

