Combined Transcriptomic and Proteomic Profiling of E. coli under Microaerobic versus Aerobic Conditions: The Multifaceted Roles of Noncoding Small RNAs and Oxygen-Dependent Sensing in Global Gene Expression Control
- PMID: 35269716
- PMCID: PMC8910356
- DOI: 10.3390/ijms23052570
Combined Transcriptomic and Proteomic Profiling of E. coli under Microaerobic versus Aerobic Conditions: The Multifaceted Roles of Noncoding Small RNAs and Oxygen-Dependent Sensing in Global Gene Expression Control
Abstract
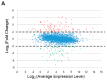
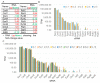
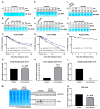
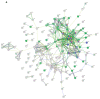
Adaptive mechanisms that facilitate intestinal colonization by the human microbiota, including Escherichia coli, may be better understood by analyzing the physiology and gene expression of bacteria in low-oxygen environments. We used high-throughput transcriptomics and proteomics to compare the expression profiles of E. coli grown under aerobic versus microaerobic conditions. Clustering of high-abundance transcripts under microaerobiosis highlighted genes controlling acid-stress adaptation (gadAXW, gadAB, hdeAB-yhiD and hdeD operons), cell adhesion/biofilm formation (pgaABCD and csgDEFG operons), electron transport (cydAB), oligopeptide transport (oppABCDF), and anaerobic respiration/fermentation (hyaABCDEF and hycABCDEFGHI operons). In contrast, downregulated genes were involved in iron transport (fhuABCD, feoABC and fepA-entD operons), iron-sulfur cluster assembly (iscRSUA and sufABCDSE operons), aerobic respiration (sdhDAB and sucABCDSE operons), and de novo nucleotide synthesis (nrdHIEF). Additionally, quantitative proteomics showed that the products (proteins) of these high- or low-abundance transcripts were expressed consistently. Our findings highlight interrelationships among energy production, carbon metabolism, and iron homeostasis. Moreover, we have identified and validated a subset of differentially expressed noncoding small RNAs (i.e., CsrC, RyhB, RprA and GcvB), and we discuss their regulatory functions during microaerobic growth. Collectively, we reveal key changes in gene expression at the transcriptional and post-transcriptional levels that sustain E. coli growth when oxygen levels are low.
Keywords: acid stress response; anaerobic respiration; iron homeostasis; transcriptional and post-transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

