Characterization of Recruited Mononuclear Phagocytes following Corneal Chemical Injury
- PMID: 35269717
- PMCID: PMC8910730
- DOI: 10.3390/ijms23052574
Characterization of Recruited Mononuclear Phagocytes following Corneal Chemical Injury
Abstract
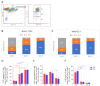
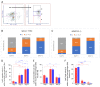
Mononuclear phagocytes (MP) have central importance in innate immunity, inflammation, and fibrosis. Recruited MPs, such as macrophages, are plastic cells and can switch from an inflammatory to a restorative phenotype during the healing process. However, the role of the MPs in corneal wound healing is not completely understood. The purpose of this study is to characterize the kinetics of recruited MPs and evaluate the role of macrophage metalloelastase (MMP12) in the healing process, using an in vivo corneal chemical injury model. Unwounded and wounded corneas of wild-type (WT) and Mmp12-/- mice were collected at 1, 3, and 6 days after chemical injury and processed for flow cytometry analysis. Corneal MP phenotype significantly changed over time with recruited Ly6Chigh (proinflammatory) cells being most abundant at 1 day post-injury. Ly6Cint cells were highly expressed at 3 days post-injury and Ly6Cneg (patrolling) cells became the predominant cell type at 6 days post-injury. CD11c+ dendritic cells were abundant in corneas from Mmp12-/- mice at 6 days post-injury. These findings show the temporal phenotypic plasticity of recruited MPs and provide valuable insight into the role of the MPs in the corneal repair response, which may help guide the future development of MP-targeted therapies.
Keywords: MMP12; corneal injury; dendritic cells; macrophages; monocytes; mononuclear phagocytes; myeloid cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K.J.N., et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

