Structure, Oligomerization and Activity Modulation in N-Ribohydrolases
- PMID: 35269719
- PMCID: PMC8910321
- DOI: 10.3390/ijms23052576
Structure, Oligomerization and Activity Modulation in N-Ribohydrolases
Abstract
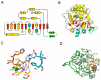
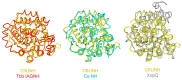
Enzymes catalyzing the hydrolysis of the N-glycosidic bond in nucleosides and other ribosides (N-ribohydrolases, NHs) with diverse substrate specificities are found in all kingdoms of life. While the overall NH fold is highly conserved, limited substitutions and insertions can account for differences in substrate selection, catalytic efficiency, and distinct structural features. The NH structural module is also employed in monomeric proteins devoid of enzymatic activity with different physiological roles. The homo-oligomeric quaternary structure of active NHs parallels the different catalytic strategies used by each isozyme, while providing a buttressing effect to maintain the active site geometry and allow the conformational changes required for catalysis. The unique features of the NH catalytic strategy and structure make these proteins attractive targets for diverse therapeutic goals in different diseases.
Keywords: N-ribohydrolases; drug design; quaternary structure; ribosides; structural enzymology.
Conflict of interest statement
The author declares no conflict of interest.
Figures






Similar articles
-
Active site plasticity revealed from the structure of the enterobacterial N-ribohydrolase RihA bound to a competitive inhibitor.BMC Struct Biol. 2010 Jun 8;10:14. doi: 10.1186/1472-6807-10-14. BMC Struct Biol. 2010. PMID: 20529317 Free PMC article.
-
Structural basis of catalysis and substrate recognition by the NAD(H)-dependent α-d-glucuronidase from the glycoside hydrolase family 4.Biochem J. 2021 Feb 26;478(4):943-959. doi: 10.1042/BCJ20200824. Biochem J. 2021. PMID: 33565573
-
Structural basis for substrate specificity in group I nucleoside hydrolases.Biochemistry. 2008 Apr 15;47(15):4418-26. doi: 10.1021/bi702448s. Epub 2008 Mar 25. Biochemistry. 2008. PMID: 18361502
-
Structural analyses reveal two distinct families of nucleoside phosphorylases.Biochem J. 2002 Jan 1;361(Pt 1):1-25. doi: 10.1042/0264-6021:3610001. Biochem J. 2002. PMID: 11743878 Free PMC article. Review.
-
Structural plasticity in the human cytosolic sulfotransferase dimer and its role in substrate selectivity and catalysis.Drug Metab Pharmacokinet. 2015 Feb;30(1):3-20. doi: 10.1016/j.dmpk.2014.10.004. Epub 2014 Nov 5. Drug Metab Pharmacokinet. 2015. PMID: 25760527 Review.
Cited by
-
Biological Catalysis and Information Storage Have Relied on N-Glycosyl Derivatives of β-D-Ribofuranose since the Origins of Life.Biomolecules. 2023 Apr 30;13(5):782. doi: 10.3390/biom13050782. Biomolecules. 2023. PMID: 37238652 Free PMC article. Review.
-
Direct Measurement of Nucleoside Ribohydrolase Enzyme Activities in Trichomonas vaginalis Cells Using 19F and 13C-Edited 1H NMR Spectroscopy.Anal Chem. 2023 Mar 28;95(12):5300-5306. doi: 10.1021/acs.analchem.2c05330. Epub 2023 Mar 14. Anal Chem. 2023. PMID: 36917470 Free PMC article.
-
Protein Oligomerization.Int J Mol Sci. 2023 Jun 26;24(13):10648. doi: 10.3390/ijms241310648. Int J Mol Sci. 2023. PMID: 37445826 Free PMC article.
-
Structure-Function Insights into the Dual Role in Nucleobase and Nicotinamide Metabolism and a Possible Use in Cancer Gene Therapy of the URH1p Riboside Hydrolase.Int J Mol Sci. 2024 Jun 27;25(13):7032. doi: 10.3390/ijms25137032. Int J Mol Sci. 2024. PMID: 39000137 Free PMC article.
-
Structure-Guided Insight into the Specificity and Mechanism of a Parasitic Nucleoside Hydrolase.Biochemistry. 2022 Sep 6;61(17):1853-1861. doi: 10.1021/acs.biochem.2c00361. Epub 2022 Aug 22. Biochemistry. 2022. PMID: 35994320 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

