Mycobacterium tuberculosis Acetyltransferase Suppresses Oxidative Stress by Inducing Peroxisome Formation in Macrophages
- PMID: 35269727
- PMCID: PMC8909987
- DOI: 10.3390/ijms23052584
Mycobacterium tuberculosis Acetyltransferase Suppresses Oxidative Stress by Inducing Peroxisome Formation in Macrophages
Abstract
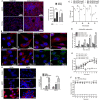
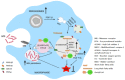
Mycobacterium tuberculosis (Mtb) inhibits host oxidative stress responses facilitating its survival in macrophages; however, the underlying molecular mechanisms are poorly understood. Here, we identified a Mtb acetyltransferase (Rv3034c) as a novel counter actor of macrophage oxidative stress responses by inducing peroxisome formation. An inducible Rv3034c deletion mutant of Mtb failed to induce peroxisome biogenesis, expression of the peroxisomal β-oxidation pathway intermediates (ACOX1, ACAA1, MFP2) in macrophages, resulting in reduced intracellular survival compared to the parental strain. This reduced virulence phenotype was rescued by repletion of Rv3034c. Peroxisome induction depended on the interaction between Rv3034c and the macrophage mannose receptor (MR). Interaction between Rv3034c and MR induced expression of the peroxisomal biogenesis proteins PEX5p, PEX13p, PEX14p, PEX11β, PEX19p, the peroxisomal membrane lipid transporter ABCD3, and catalase. Expression of PEX14p and ABCD3 was also enhanced in lungs from Mtb aerosol-infected mice. This is the first report that peroxisome-mediated control of ROS balance is essential for innate immune responses to Mtb but can be counteracted by the mycobacterial acetyltransferase Rv3034c. Thus, peroxisomes represent interesting targets for host-directed therapeutics to tuberculosis.
Keywords: Mycobacterium tuberculosis; Rv3034c; acetyltransferase; macrophages; oxidative stress; peroxisome.
Conflict of interest statement
The authors have no conflict of interest.
Figures



References
-
- World Health Organization . Global Tuberculosis Report 2020. World Health Organization; Geneva, Switzerland: 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

