Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders
- PMID: 35269741
- PMCID: PMC8910759
- DOI: 10.3390/ijms23052591
Damage-Associated Molecular Patterns (DAMPs) in Retinal Disorders
Abstract
Damage-associated molecular patterns (DAMPs) are endogenous danger molecules released from the extracellular and intracellular space of damaged tissue or dead cells. Recent evidence indicates that DAMPs are associated with the sterile inflammation caused by aging, increased ocular pressure, high glucose, oxidative stress, ischemia, mechanical trauma, stress, or environmental conditions, in retinal diseases. DAMPs activate the innate immune system, suggesting their role to be protective, but may promote pathological inflammation and angiogenesis in response to the chronic insult or injury. DAMPs are recognized by specialized innate immune receptors, such as receptors for advanced glycation end products (RAGE), toll-like receptors (TLRs) and the NOD-like receptor family (NLRs), and purine receptor 7 (P2X7), in systemic diseases. However, studies describing the role of DAMPs in retinal disorders are meager. Here, we extensively reviewed the role of DAMPs in retinal disorders, including endophthalmitis, uveitis, glaucoma, ocular cancer, ischemic retinopathies, diabetic retinopathy, age-related macular degeneration, rhegmatogenous retinal detachment, proliferative vitreoretinopathy, and inherited retinal disorders. Finally, we discussed DAMPs as biomarkers, therapeutic targets, and therapeutic agents for retinal disorders.
Keywords: DAMPs; age-related macular degeneration; diabetic retinopathy; endophthalmitis; glaucoma; inherited retinal disorders; ischemic retinopathies; ocular cancer; proliferative vitreoretinopathy; uveitis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
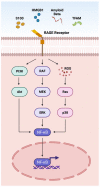
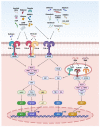
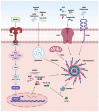
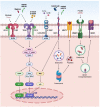
References
-
- Lim R.R., Vaidya T., Gadde S.G., Yadav N.K., Sethu S., Hainsworth D.P., Mohan R.R., Ghosh A., Chaurasia S.S. Correlation between systemic S100A8 and S100A9 levels and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019;13:1581–1589. doi: 10.1016/j.dsx.2019.03.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

