Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery
- PMID: 35269753
- PMCID: PMC8910138
- DOI: 10.3390/ijms23052612
Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery
Abstract
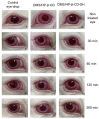
The goal of this study was the design and evaluation of a thiolated cyclodextrin providing high drug solubilizing and mucoadhesive properties for ocular drug delivery. Hydroxypropyl-β-cyclodextrin (HP-β-CD) was thiolated via a microwave-assisted method, resulting in a degree of thiolation of 33%. Mucoadhesive properties of thiolated HP-β-CD (HP-β-CD-SH) were determined via rheological measurements and ex vivo studies on isolated porcine cornea. Due to thiolation of HP-β-CD, a 2-fold increase of mucus viscosity and a 1.4-fold increase in residence time on isolated corneal tissue were achieved. After instillation, the mean precorneal residence time and AUC of dexamethasone (DMS) eye drops were 4-fold and 11.7-fold enhanced by HP-β-CD-SH, respectively. Furthermore, in the presence of HP-β-CD-SH, a constant high level of DMS in aqueous humour between 30 and 150 min after administration was observed. These results suggest that HP-β-CD-SH is an excellent excipient for ocular formulations of poorly soluble drugs in order to prolong their ocular residence time and bioavailability.
Keywords: aqueous humour; dexamethasone; drug delivery; hydroxypropyl-β-cyclodextrin; mucoadhesive; ocular; thiolation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

